In this issue of Blood, Dumontet et al1 highlight the role of cancer cell-derived extracellular vesicles in forming a tumor supportive stromal cell niche in follicular lymphoma.
Extracellular vesicles are small membrane-encapsulated vesicles that harbor important information such as RNA and proteins derived from their cell of origin. Their role in priming the premetastatic niche has been well recognized in solid cancers. This is exemplified by their ability to induce endothelial permeability and recruit bone marrow progenitor cells and by driving metastasis formation in an organotrophic manner.2,3 In hematologic malignancies, cancer cell–derived extracellular vesicles are equally important in the generation of a tumor-supportive microenvironment. Among others, they have been reported to mediate the transition of stromal cells to cancer-associated fibroblasts in chronic lymphocytic leukemia.4
Follicular lymphoma is an indolent lymphoma with lymph node and bone marrow involvement, the latter presenting an adverse risk factor and being crucially dependent on active homing of malignant cell subclones to the bone marrow niche.5,6 However, cell–cell communication networks, which are involved in the selection and homing of certain malignant B cell subclones to the bone marrow, remain to be explored in more detail. Similarly, the role of extracellular vesicles in forming the bone marrow niche in follicular lymphoma is an important area of current investigation.
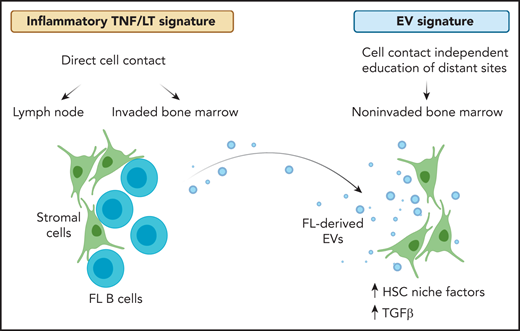
In the presented work, Dumontet et al introduce cancer cell–derived extracellular vesicles as an important player within the follicular lymphoma bone marrow microenvironment (see figure). The authors isolate extracellular vesicles from conditioned media of follicular lymphoma cell lines, primary follicular lymphoma B cells, and patient-derived blood plasma. Using healthy donor–derived mesenchymal stromal cells, they identify a rapid uptake of extracellular vesicles in stromal cells and a vesicle-mediated increase in stromal cell–mediated survival support for follicular lymphoma cells. Given prior reports on lymphoid-like stromal cell differentiation induced by stimulation with tumor necrosis factor-α and lymphotoxin-α1β2 (TNF/LT), the authors put vesicle-mediated effects (EV signature) in context with TNF/LT-triggered alterations in stromal cells. They successfully dissect differential gene expression profiles of the 2 lymphoma-supportive stromal cell stimuli. Of note, there is limited overlap between EV- and TNF/LT-induced gene expression signatures in stromal cells. Comparing observed gene expression changes to relevant tissue sites for disease manifestation, the TNF/LT signature shows enrichment in mesenchymal stromal cells of lymphoma cell–invaded lymph node and bone marrow sites, whereas the EV signature shows enrichment in mesenchymal stromal cells of patient-derived noninvaded bone marrow. Similarly, coculture assays of malignant B cells with mesenchymal stromal cells show an overlap with expression changes observed in invaded bone marrow. This indicates that the TNF/LT signature resembles pathologically relevant alterations of the tumor microenvironment where stromal cells are in close contact with follicular lymphoma cells. On the other hand, vesicle-mediated effects overlap with stromal cell subsets in the bone marrow that resemble components of the hematopoietic stem cell niche. Thus, it is likely that priming of the bone marrow site via extracellular vesicles allows for later infiltration of follicular lymphoma cells. The authors provide further evidence pointing to a vesicle-induced expression of genes centrally important for the formation and maintenance of the hematopoietic stem cell niche and osteolineage and adipogenic differentiation. Focusing on signaling alterations triggered by extracellular vesicles, a dominant role for transforming growth factor β (TGFβ) becomes apparent. As such, higher levels of TGFβ1 and TGFβ3 in vesicle-primed mesenchymal stromal cells are reported. A detailed characterization of transcription factors encapsulated by extracellular vesicles or driving the EV signature in stromal cells points to a central role of the TGFβ signaling pathway. Follicular lymphoma–derived extracellular vesicles trigger canonical TGFβ-SMAD and noncanonical TGFβ-p38 signaling: both are proven reversible by the TGFBR1 inhibitor galunisertinib. In addition, TGFβ-independent enrichment for signal transducer and activator of transcription 6 is observed in follicular lymphoma–derived extracellular vesicles.
Gene expression alterations in stromal cells vary across tissue sites of disease manifestation in follicular lymphoma. At sites of direct cell contact, namely lymph node and invaded bone marrow, an inflammatory TNF/LT-mediated expression signature in stromal cells provides survival support for malignant B cells. In addition, follicular lymphoma cells release extracellular vesicles that can prime stromal cells at distant sites in a cell contact–independent manner. Thus, gene expression in noninvaded bone marrow stromal cells derived from follicular lymphoma patients correlate with EV-mediated alterations. The latter is characterized by an upregulation of HSC niche factors and TGFβ signaling in stromal cells. FL, follicular lymphoma; HSC, hematopoietic stem cell. Created with BioRender.com.
Gene expression alterations in stromal cells vary across tissue sites of disease manifestation in follicular lymphoma. At sites of direct cell contact, namely lymph node and invaded bone marrow, an inflammatory TNF/LT-mediated expression signature in stromal cells provides survival support for malignant B cells. In addition, follicular lymphoma cells release extracellular vesicles that can prime stromal cells at distant sites in a cell contact–independent manner. Thus, gene expression in noninvaded bone marrow stromal cells derived from follicular lymphoma patients correlate with EV-mediated alterations. The latter is characterized by an upregulation of HSC niche factors and TGFβ signaling in stromal cells. FL, follicular lymphoma; HSC, hematopoietic stem cell. Created with BioRender.com.
Altogether, the authors generate new insights regarding the different expression states of bone marrow stromal cells across sites of disease manifestation. They successfully dissect driving forces for the respective alterations, focusing on cell contact, TNF/LT-mediated vs cell contact–independent, extracellular vesicle–mediated stimulation. The relevance of context-dependent remodeling of the tumor microenvironment is addressed by highlighting differential cell–cell communication networks that are established between follicular lymphoma cells and bone marrow stromal cells primed with TNF/LT or vesicle stimuli, respectively. Differences between lymph node and bone marrow–located follicular lymphoma cells indicate the bone marrow niche as a site that harbors malignant B cells that are less proliferatively active but show the expression of drug resistance–associated genes.
Understanding tumor microenvironment alterations that allow the dissemination of malignant cells throughout the body and foster their survival is of utmost importance to design rational treatment strategies. Recent success of ibrutinib as a pathway-targeted inhibitor in hematologic malignancies has emphasized the benefit of inhibiting malignant B cells and simultaneously limiting their access to a tumor supportive microenvironment.7 As such, ibrutinib treatment results in a release of malignant cells from secondary tissue sites into the peripheral blood in chronic lymphocytic leukemia: a phenomena that potentiates treatment success.7 Extracellular vesicle–mediated TGFβ signaling in bone marrow mesenchymal stromal cells, as presented by the authors, may represent another important, targetable axis in the establishment of a cancer cell favorable tissue niche and may impact treatment response in follicular lymphoma.
Similarly, TGFβ-positive stromal cells have recently been associated with resistance to cancer immunotherapy.8 A role of extracellular vesicles in forming a tissue niche that provides protection of cancer cells from targeted therapy and immunotherapy may be important to explore in the future, and new insight may guide the design of combinatorial treatment strategies.
Conflict-of-interest disclosure: The author declares no competing financial interests.