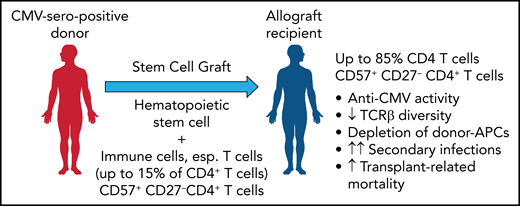
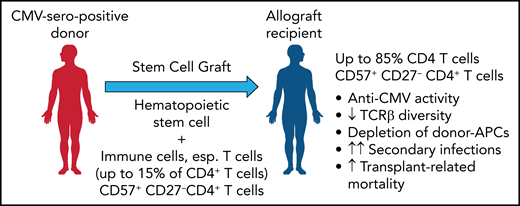
In this issue of Blood, Yeh et al found that cytomegalovirus (CMV) drives the long-term expansion of donor CD4 cytotoxic T lymphocytes (CD57+/CD27−) after allogeneic stem cell transplant (SCT). This effect was associated with both a profound impairment in immune repertoire diversity and a loss of donor antigen-presenting cells (APCs) in grafts from seropositive donors. CMV may thus lead to a much broader defect in pathogen-specific immunity than previously thought, which would explain the higher transplant-related mortality in SCT recipients who have CMV-seropositive donors.1
Despite advances in antiviral prophylaxis and treatment, positive donor CMV serological status remains an important risk factor for increased transplant-related complications and mortality.2 The higher incidence of secondary bacterial and fungal infections in patients receiving their stem cell graft from a CMV-seropositive donor points to CMV-related defects in pathogen-specific immunity .3
CMV reactivation and the myelotoxic and lymphotoxic side effects of long-term antiviral chemotherapy with ganciclovir or valganciclovir are well documented to induce long-term immune deficiency. These side effects not only foster recurrent CMV infections and disease but also increase the incidence of secondary bacterial and fungal infections associated with increased transplant-related mortality.4,5 In addition, CMV reactivation after allogeneic SCT skews the immune repertoire by a sizable expansion of effector memory T cells and a reduction in the overall T-cell receptor β (TCR-β) diversity, thus altering both CD4+ and CD8+ T-cell reconstitution.6,7 Interestingly, the negative association between donor CMV seropositivity and long-term clinical outcome after allogeneic SCT has been demonstrated to be independent of the extent of CMV reactivation and disease.2,8,9
As a likely cause, the results demonstrate that receiving a graft from a CMV-seropositive donor is associated with a significant expansion of a highly differentiated, cytotoxic effector memory T-cell subset characterized by the expression of CD57 (see figure). This expansion was found to be strongly dependent on the CMV serostatus of donor and recipient but independent of CMV reactivation and to persist over many years after allogeneic SCT. While this expanded fraction of CD57+/CD27− T-cells in both the CD4 and CD8 compartments comprised up to 15% of T cells in healthy CMV seropositive individuals, it increased beyond 70% or even 85% in CMV seronegative recipients of a CMV seropositive stem cell graft. This expanded CD57+/CD27− CD4+ and, less impressively also a CD8+ T-cell subset expressed an effector memory phenotype (TCR7−/CD45RAlow-intCD45RO/CD28−) with low expression of exhaustion/inhibitory markers and showed upregulated activation markers and increased expression of granzyme B, as well as the Th1 cytokines tumor necrosis factor and interferon γ. Stimulation with pp65 peptide pools and both degranulation analysis and cytokine profiling showed that 1% to 15% of the CD57+/CD27− CD4+ T cells were found to be reactive to pp65. Among the CMV tetramer-positive CD4+ T cells, most were CD57+/CD27−, in contrast to the CMV tetramer-negative CD4+ T cells. It would be interesting to clarify whether these are predominantly donor graft–derived cells or are generated de novo after transplantation.
By transferring a donor-derived, expanded CD57+ 27− CD4+ T-cell population from a CMV-seropositive donor that further expands in the allograft recipient, T-cell inflation is induced with a reduced TCR repertoire and a depletion of donor APCs. This result increases the risk of secondary infections and transplant-related mortality. Professional illustration by Patrick Lane, ScEYEnce Studios.
By transferring a donor-derived, expanded CD57+ 27− CD4+ T-cell population from a CMV-seropositive donor that further expands in the allograft recipient, T-cell inflation is induced with a reduced TCR repertoire and a depletion of donor APCs. This result increases the risk of secondary infections and transplant-related mortality. Professional illustration by Patrick Lane, ScEYEnce Studios.
Neither acute or chronic graft versus host disease, nor treatment with calcineurin inhibitors or steroids had any impact on the expansion of this CD57+/CD27− CD4+ T-cell population. In contrast, CMV reactivation was found to increase this fraction, whereas donor seropositivity for other herpes viruses, specifically Epstein-Barr virus, had no impact.
CD8 T-cell “memory inflation” occurs in healthy CMV-seropositive individuals without evidence of CMV disease. Therefore, it is not surprising that the expansion of the CD57+/CD27− T cells also occurred in CMV-seropositive graft/CMV-seronegative SCT recipients, even in the absence of detectable CMV reactivation. Further experiments are needed to clarify the role of CD4+ and CD8+ T cells with the CD57+/CD27− phenotype in controlling or suppressing CMV reactivation and whether subclinical CMV reactivations drive their long-term expansion and persistence.
Of note, the expansion of this subset was accompanied by a significant loss in both CD4 and CD8 T-cell (but not B-cell) receptor diversity that may compromise future adaptive immune control of CMV+ graft recipients. Furthermore, the expansion of cytotoxic CD57+/CD27− CD4+ T cells was associated with a significant reduction of major histocompatibility complex class II–expressing monocytes. Further experiments are needed to demonstrate whether the late effector memory CD57+/CD27− CD4+ T cells with their high cytolytic activity (high expression of granzyme B and CD107a) induce this marked loss of APCs (and may be of CMV-infected, donor-derived APCs).
In summary, the data provided herein indicate that CMV is responsible for a much broader defect in pathogen-specific immunity and emphasize the question of whether CMV− recipients should receive a transplant from a CMV+ donor. The underlying virological and immunological mechanisms warrant extensive interrogations into preclinical systems to ascertain the true cause-and-effect relationships.
Conflict-of-interest disclosure: The authors declare no competing financial interests.