Key Points
We observed bidirectional increased risks between BCL and TCL, with estimated risks nearly fivefold higher than the general population.
The magnitude of these associations varied substantially by lymphoma subtype, supporting etiologic heterogeneity among different subtypes.
Abstract
Lymphoma survivors have a significantly higher risk of developing second primary lymphoma than the general population; however, bidirectional risks of developing B- and T-cell lymphomas (BCLs and TCLs) specifically are less well understood. We used population-based cancer registry data to estimate the subtype-specific risks of second primary lymphoma among patients with first BCL (n = 288 478) or TCL (n = 23 747). We observed nearly fivefold increased bidirectional risk between BCL and TCL overall (TCL following BCL: standardized incidence ratio [SIR] = 4.7, 95% confidence interval [CI] = 4.2-5.2; BCL following TCL: SIR = 4.7, 95% CI = 4.1-5.2), but the risk varied substantially by lymphoma subtype. The highest SIRs were observed between Hodgkin lymphoma (HL) and peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) (PTCL-NOS following HL: SIR = 27.5; HL following PTCL-NOS: SIR = 31.6). Strikingly elevated risks also were notable for angioimmunoblastic T-cell lymphoma (AITL) and diffuse large B-cell lymphoma (DLBCL) (AITL following DLBCL: SIR = 9.7; DLBCL following AITL: SIR = 15.3). These increased risks were strongest within the first year following diagnosis but remained persistently elevated even at ≥5 years. In contrast, SIRs were <5 for all associations of TCL with chronic lymphocytic leukemia/small lymphocytic lymphoma and follicular lymphoma. These patterns support etiologic heterogeneity among lymphoma subtypes and provide further insights into lymphomagenesis.
Introduction
Patients with lymphoma have a significantly higher risk of developing subsequent solid tumors and hematologic neoplasms than the general population.1,2 Prior studies have identified an increased risk of Hodgkin lymphoma (HL) following non-Hodgkin lymphoma (NHL) overall2 and following subtype-specific B-cell lymphomas (BCLs).1 In addition, patients treated for HL have been found to have an elevated risk of subsequent NHL overall.3
In contrast, the bidirectional risks of BCL and T-cell lymphomas (TCLs) are less well understood. Survivors of cutaneous T-cell lymphoma (CTCL) have been reported to experience increased risks of subsequent HL, chronic lymphocytic leukemia (CLL), and other NHLs.4,5 Additionally, case series have described subtype-specific BCL following subtype-specific TCL and conversely subtype-specific TCL following subtype-specific BCL.6-8 However, bidirectional risks of BCL and TCL have not been comprehensively assessed in a large-scale population-based study with systematic ascertainment of multiple primary malignancies. We leveraged the Surveillance, Epidemiology, and End Results (SEER) Program to assess subtype-specific bidirectional risks of BCL and TCL and whether shared etiologies between lymphoma-specific subtypes might exist.
Study design
Study cohort
The SEER Program collects information on incident cancer cases, including patient demographics, vital status, and details surrounding cancer diagnosis and treatment (eg, primary site, morphology, stage, and initial course of treatment) among residents of participating cancer registry areas, as described elsewhere.9 Using data from 17 SEER registry areas that cover nearly 28% of the US population,10 we identified patients diagnosed with first primary BCL (n = 288 478) or first primary TCL (n = 23 747; Table 1) during 2000-2016 who were followed until date of diagnosis of a second primary cancer, death, lost to follow-up, or end of study (31 December 2017), whichever occurred first.
Statistical analyses
We quantified the risk of second primary lymphoma (BCL or TCL) using standardized incidence ratios (SIRs), comparing the number of observed (Obs.) second primary lymphoma cases after first primary lymphoma with that expected in the general population (SEER*Stat, version 8.3.8).9 The expected number of second primary lymphoma cases was calculated by multiplying the incidence rate of each first primary BCL or TCL in the general population stratified by age (5-year groups), sex, race (White, Black, other), and calendar year by the person-time at risk. We first conducted analyses of BCL and TCL overall and then undertook subtype-specific analyses limited to the 5 most commonly occurring BCL and TCL subtypes. Exploratory, subtype-specific analyses stratified by sex, age, and latency were also performed when case count allowed. All statistical tests were 2 sided, and P < .05 was considered statistically significant.
Results and discussion
We observed nearly fivefold increased bidirectional risk between BCL and TCL (Table 1; TCL following BCL (Obs., 354; SIR, 4.7; 95% CI, 4.2-5.2; BCL following TCL: Obs., 300; SIR, 4.7; 95% CI, 4.1-5.2). The elevated risks of second primary lymphoma were persistent across age groups (age <60 and ≥60 years), sex, latency (within 1, 1-4, and ≥5 years) and different time periods (2000-2005, 2006-2011, and 2012-2016). The highest SIRs were observed in the first year after first primary lymphoma diagnosis but remained statistically significantly elevated at ≥5 years.
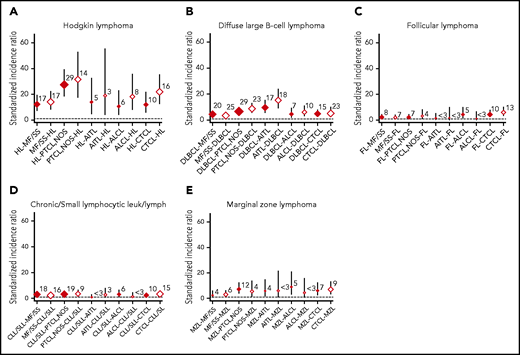
Risks by BCL and TCL subtypes are shown in Figure 1 and supplemental Table 1 (available on the Blood Web site). The subtype-specific risk patterns tended to be bidirectional, with increased risks observed most notably (SIR >10) between HL and all TCL subtypes. The highest SIRs were observed for HL and peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), including PTCL-NOS following HL (SIR, 27.5; 95% CI, 18.4-39.4) and HL following PTCL-NOS (SIR, 31.6; 95% CI, 17.3-53.0). We also observed significant bidirectional more than fivefold increased risks between diffuse large B-cell lymphoma (DLBCL) and PTCL-NOS, angioimmunoblastic T-cell lymphoma (AITL), and CTCL. High SIRs were particularly prominent between DLBCL and AITL (AITL following DLBCL, SIR, 9.7; 95% CI, 5.7-15.5; DLBCL following AITL, SIR, 15.3; 95% CI, 9.1-24.2). Also, bidirectional increased risks were more than fivefold between marginal zone lymphoma (MZL) and PTCL-NOS and CTCL, but the number of second lymphoma cases was limited. In contrast, SIRs for all TCL subtypes and CLL/small lymphocytic lymphoma (SLL) or follicular lymphoma (FL) were <5, with the exception of FL after CTCL (SIR, 6.0; 95%CI 3.2-10.3). SIRs of all BCL subtypes except HL also were <5 in mycosis fungoides/Sezary syndrome (MF/SS). Estimated subtype-specific SIRs stratified by sex, age, and latency were limited by the number of patients with second primary lymphoma in each group, but the patterns remained the same regardless of sex, age, and latency (supplemental Tables 2-4).
SIRs with 95% CIs for the reciprocal associations between BCL and TCL. (A) HL. (B) DLBCL. (C) FL. (D) CLL/SLL. (E) MZL. Numbers shown represent the observed cases of second primary lymphoma. Red diamonds correspond to TCL following BCL, and white diamonds correspond to BCL following TCL. leuk/lymph, leukemia/lymphoma.
SIRs with 95% CIs for the reciprocal associations between BCL and TCL. (A) HL. (B) DLBCL. (C) FL. (D) CLL/SLL. (E) MZL. Numbers shown represent the observed cases of second primary lymphoma. Red diamonds correspond to TCL following BCL, and white diamonds correspond to BCL following TCL. leuk/lymph, leukemia/lymphoma.
This study is the first to assess reciprocal risks of subtype-specific associations of BCLs and TCLs in a large-scale, population-based study of lymphoma survivors. We found a bidirectional increased risk between BCL and TCL; however, the strength of association depended on the specific lymphoma subtype, with particularly high reciprocal risks for HL, DLBCL, and MZL among the BCLs and for PTCL-NOS and AITL among the TCLs. The increased bidirectional risks were strongest within 1 year after first lymphoma, raising the possibility of a surveillance effect or subsequently diagnosed composite lymphoma. These results suggest that there may be a shared predisposition and/or exposure(s) leading to the increased reciprocal risk of specific lymphoma subtypes.
Previous research provides some insights into potential risk factors that could explain the risk patterns we observed.11-14 In the pooled analysis examining medical history, family history, lifestyle, and occupation, the greatest difference in risk factors occurred between BCL and TCL; however, PTCL clustered with BCL in some hierarchical analyses.12 Immunocompromised individuals, such as those living with HIV or recipients of solid-organ transplant, have an increased risk of developing BCLs and TCLs, such as HL, DLBCL, MZL, PTCL-NOS, and anaplastic large cell lymphoma, but not FL, CLL, or MF/SS.15,16 Epstein-Barr virus infection is also associated with BCLs and TCLs, including HL, DLBCL, MZL, PTCL-NOS, and AITL.17 Patterns of increased reciprocal risks in the same subtypes we observed in this study suggest that clinically apparent and/or subclinical impairment in the immune system may play a role in the bidirectional BCL-TCL associations. This hypothesis is further supported by previous studies that demonstrate that patients with DLBCL and HL without known immunodeficiency have an underlying immune dysfunction that increases incidence of infectious and autoimmune diseases after a lymphoma diagnosis.18 Intriguingly, shared genetic aberrations also could contribute to the reciprocal risks we observed. For example, aberrations in TET2 have been demonstrated in several lymphoma subtypes, including DLBCL, PTCL-NOS and AITL,19 and clonal hematopoiesis could lead to shared somatic pathogenic genetic aberrations seen both in BCL and TCL, such as JAK/STAT pathway mutations.20 Our results support the importance of further research into characterization of patients with multiple primary lymphomas to further understand lymphomagenesis in various subtypes.
Our findings of reciprocal associations in BCL and TCL should be interpreted in the context of several limitations. Prior studies found that change in diagnosis occurred in ∼5% of cases by central review.21,22 Laurent et al showed that change in diagnosis from mature BCL to TCL or vice versa was rare (0.1% of cases) and therefore is unlikely to have affected our primary findings. However, we cannot exclude the possibility of misclassification of lymphoma subtypes in the absence of a centralized pathology review.
In summary, we found increased bidirectional risks of BCLs and TCLs, but the magnitude of these associations varied substantially by lymphoma subtype, confirming etiologic heterogeneity and shared risks among different subtypes. Further epidemiologic investigations with detailed, individual-level data on treatments and lymphoma risk factors, focusing on host immune system characteristics, comorbid conditions, and their associated immunomodulatory treatments, status of relevant infections, and genetic predisposition, may reveal new insights in lymphomagenesis.
Acknowledgments
The authors thank the staff of the SEER Program in the creation of the database.
This study was supported by the Intramural Program of the National Cancer Institute, National Institutes of Health, US Department of Health and Human Services.
The opinions and information in this article are those of the authors and do not represent the views and/or policies of the US Food and Drug Administration.
Authorship
Contribution: D.C., G.M.D., and L.M.M. designed the research and analyzed data; and D.C., G.M.D., C.R.F., and L.M.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dai Chihara, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX; e-mail: dchihara@mdanderson.org.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.