Abstract
Introduction
It is well established that von Willebrand factor (VWF) levels increase with age among healthy adults. Recently, there is emerging research demonstrating this may also occur in patients with von Willebrand disease (VWD), particularly type 1 VWD, and may be related to comorbidities. Despite increasing VWF levels, it remains unclear as to whether or not this alters bleeding phenotype. It is also unclear why this occurs most commonly in patients with type 1 VWD, but VWF mutation status may play a role.
Older patients commonly undergo invasive procedures, and all VWD patients require periprocedural VWD-specific therapy to ensure appropriate hemostasis. If older type 1 VWD patients have experienced normalization of VWF levels, and no longer have an increased risk of bleeding, VWD-specific therapy may increase thrombosis risk, especially among patients with underlying cardiovascular disease or related risk factors, subject the patient to other adverse reactions such as hyponatremia, and is unnecessarily costly. For these reasons, investigation into the effect of age on VWF levels and bleeding risk in type 1 VWD patients is sorely needed.
Methods
This is an NHLBI-funded K23 multicenter, cross-sectional study to determine the effect of age on VWF levels and bleeding risk in patients with type 1 VWD, and to determine if pathogenic VWF mutations alter this effect.Individuals with a new or historical diagnosis of type 1 VWD (defined as clinical symptoms consistent with VWD and VWF antigen level or ristocetin cofactor activity <0.50 IU/mL) and age 18 or older are enrolled during routine clinic visits at participating Hemophilia Treatment Centers (HTCs). Following enrollment, pertinent medical history is obtained; the condensed MCMDM-1 VWD Bleeding Assessment Tool is administered, with bleeding history based on bleeding symptoms during the past 5 years; and blood samples are collected for the following: VWF antigen (VWF:Ag) level, VWF ristocetin cofactor activity, factor VIII activity, blood type, and VWF gene sequencing.
We hypothesize age is associated with increased VWF:Ag levels and lower condensed MCMDM-1 VWD bleeding scores in patients with type 1 VWD, and this association is weaker among those with a pathogenic VWF mutation. In addition, we hypothesize multimorbidity partially explains the association between age and VWF:Ag levels, and VWF:Ag levels partially explain the association between age and condensed MCMDM-1 VWD bleeding scores in patients with type 1 VWD. A sample size of 250 participants provides 90% power to detect an effect size of Beta=±0.032 points per year of age, which is much smaller than the observed effect size, Beta=-0.080, from preliminary data. The primary analyses will be based on multivariable linear regression models with adjustment for blood type O, exogenous estrogen therapy, multimorbidity (defined as 2 or more of the core set of 20 chronic conditions, i.e., cancer, hypertension, stroke, etc., as selected by the United States Department of Health and Human Services), and medications (aspirin, nonsteroidal antiinflammatory drugs, and anticoagulants). In the regression models, two-sided t-tests will be used with an alpha=0.05.
Results
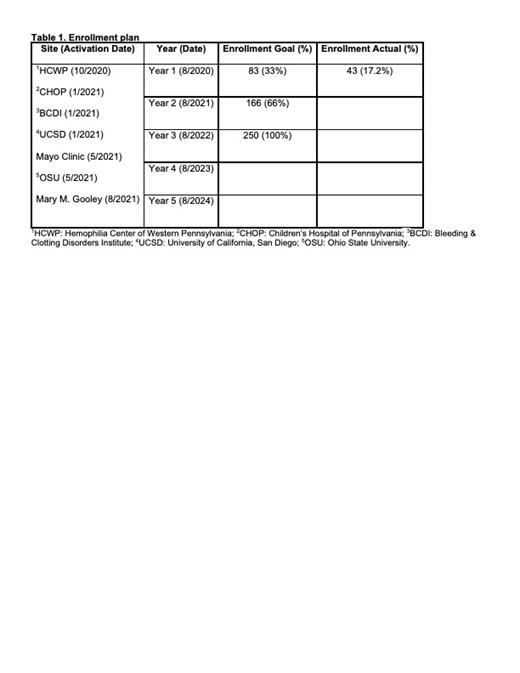
This multicenter, cross-sectional study consists of seven HTCs: Hemophilia Center of Western Pennsylvania, Children's Hospital of Pennsylvania, Mary M. Gooley Hemophilia Center, Ohio State University, Bleeding & Clotting Disorders Institute, Mayo Clinic, and University of California, San Diego. During the first year of the study, site initiation visits were conducted, regulatory approval obtained, and contracts executed. Delays in these activities occurred in large part due to the COVID-19 pandemic. As the first year of the study concludes, all sites are now active and enrolling participants. Thus far, 43 participants have been enrolled (Table 1). No barriers to enrollment have been encountered and very few patients have declined study participation.
Discussion
In conclusion, this ongoing multicenter, cross-section study seeks to determine the effect age has on VWF levels and bleeding risk in patients with type 1 VWD while exploring the role of VWF mutations and multimorbidity in this process. The results will be used to justify a longitudinal study, which is the ideal approach to research the effects of aging in this population.
Seaman: Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; HEMA Biologics: Consultancy, Honoraria. Ragni: Bioverativ (Sanofi): Membership on an entity's Board of Directors or advisory committees; Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees; Takeda Therapeutics: Membership on an entity's Board of Directors or advisory committees; Alnylam (Sanofi): Membership on an entity's Board of Directors or advisory committees; University of Pittsburgh: Research Funding; BioMarin Pharmaceutical: Membership on an entity's Board of Directors or advisory committees. Kraut: Uniqure: Consultancy. Pruthi: CSL Behring: Honoraria; Bayer Healthcare AG: Honoraria; Instrumentation Laboratory: Honoraria; Genentech: Honoraria; HEMA Biologics: Honoraria; Merck: Honoraria. Raffini: CSL Behring: Consultancy; HEMA Biologics: Consultancy; Genentech: Consultancy; Bayer: Consultancy; XaTek: Consultancy. Roberts: Takeda; Speakers Bureau: Novo Nordisk, Octapharma, Sanofi, Takeda.: Research Funding; Genentech, Novo Nordisk, Octapharma, Pfizer, Sanofi, Takeda, uniQure: Consultancy. von Drygalski: Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; uniQure: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biomarin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Research Funding; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hematherix, Inc: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Super FVa.