Abstract
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is increasingly being utilized in patients with sickle cell disease (SCD) to prevent progressive organ dysfunction, including for primary or secondary stroke prevention. However, outcomes after HCT have mostly been evaluated using broad measures such as overall survival and event free survival with limited direct physiologic evidence of improved cerebral hemodynamics. Cerebral blood flow (CBF; mL blood/100 g tissue/min) is increased in SCD to compensate for chronic anemia, reduced oxygen carrying capacity and to maintain adequate oxygen delivery to the brain tissue. In SCD patients, CBF is inversely associated with intelligence quotient (Strouse JJ, et al. Blood. 2006;108(1):379-381.) and working memory (Prussien KV, et al. Stroke. 2021;52(5):1830-1834.) and has been shown to improve with blood transfusions (Guilliams KP, et al. Blood. 2018;131(9):1012-1021.). Assessment of CBF may help predict long-term neurocognitive outcomes in patients with SCD and assess therapeutic responses to therapies. Additionally, the high metabolic demand of the brain potentially makes assessment of CBF a sensitive predictor of future multiorgan damage in patients with SCD and hence may be useful to help determine eligibility of patients for curative therapies.
Methods
We performed anatomical and hemodynamic magnetic resonance imaging (MRI) of the brain prior to, and at 6 months after HCT in children with SCD undergoing a reduced intensity conditioning based HCT on a clinical study (NCT04362293). All patients received pretransplant conditioning with hydroxyurea, azathioprine, alemtuzumab, thiotepa and low dose total body irradiation (200-400 cGy). All patients received an unmanipulated mobilized peripheral blood derived hematopoietic stem and progenitor cell graft. One patient who received the graft from a haploidentical (HAPLO) donor also received post-transplant cyclophosphamide. Graft versus host disease prophylaxis comprised of sirolimus. A 3-dimensional (3D) T1 sequence was used for gray/white matter segmentation. 3D-pulsed arterial spin labeling (ASL) perfusion imaging (3 mm isotropic) was acquired to measure resting CBF. Mean CBF values were calculated from gray matter. Mean ± standard deviation and median values for various variables are presented.
Results
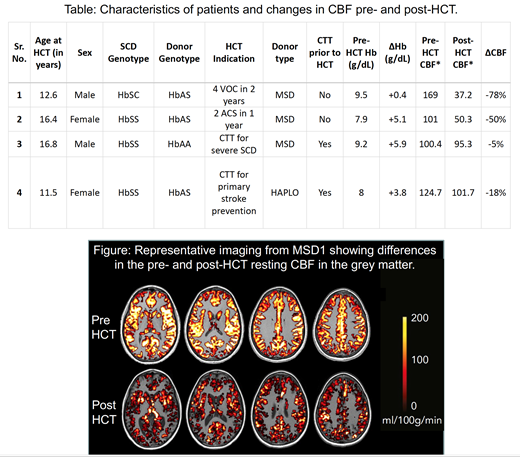
Four consecutive patients had 2 serial MRIs performed (one before HCT and another 6 months after HCT). Median age at HCT was 14.5 years. Indications for HCT, donor type and whether patients were receiving chronic transfusion therapy (CTT) prior to HCT are indicated in the Table. No patient had prior evidence of overt stroke. All patients engrafted and had >99% donor myeloid chimerism at the time of the post-HCT imaging. Mean pre-HCT hemoglobin was 8.7 ± 0.8 g/dL (median 8.6 g/dL) and it increased to 12.5 ± 2.1 g/dL (median 12.4 g/dL) at 6 months after HCT. All patients exhibited elevated resting CBF values prior to HCT (123.8 ± 32.2 mL blood/100 g tissue/min, median 112.8 mL blood/100 g tissue/min), that decreased following HCT (71.1 ± 32.1 mL blood/100 g tissue/min, median 72.8 mL blood/100 g tissue/min) (Table and Figure). None of the patients had any clinical neurological complications or imaging evidence of new infarcts or anatomical abnormalities in the follow up period.
Conclusion
Our preliminary results indicate that it is feasible to quantify CBF in patients with SCD before and after HCT using MRI. We demonstrate that cerebral hemodynamics improve after HCT in children with SCD, indicated by a decrease in CBF to near normal values (normal CBF ~80 mL blood/100 g tissue/min). The more substantial changes (-50% and -78%) are seen in children who were not receiving CTT, suggesting that CTT might partially mitigate CBF elevation prior to HCT. Nevertheless, 2 patients receiving CTT prior to HCT also had a further modest decrease in CBF after HCT (-5% and -18%). We plan to follow these changes at serial time points annually after HCT and correlate them with changes in neurocognitive functioning and other functional MRI measures. Improved CBF is expected to reduce stroke risk and may also preserve or improve neurocognitive functioning, and prospective monitoring of these outcomes are underway.
Data on all the study participants who have undergone an HCT and had 2 serial MRIs performed by the ASH annual meeting will be presented.
Sharma: Vertex Pharmaceuticals/CRISPR Therapeutics: Other: Salary support paid to institution; Medexus Inc: Consultancy; CRISPR Therapeutics: Other, Research Funding; Novartis: Other: Salary support paid to institution; Spotlight Therapeutics: Consultancy; Vindico Medical Education: Honoraria. Triplett: Miltenyi: Other: Travel, meeting registration. Hankins: Vindico Medical Education: Consultancy; UpToDate: Consultancy; Global Blood Therapeutics: Consultancy; Bluebird Bio: Consultancy.