Abstract
Background
Patients with hematologic malignancies have poor outcomes from COVID infection with associated mortality of up to 30-40%. Studies have shown that these patients are less likely to mount an antibody response after COVID infection 1. The Pfizer-BioNTech and Moderna COVID mRNA vaccines have been shown to be 94% effective in preventing severe disease in the general population. There is limited data on the efficacy of these vaccines in lymphoma patients, and to suggest the optimal timing of vaccination to elicit immunity in patients receiving immunochemotherapy.
Methods
This is a retrospective study of adult lymphoma patients who received the COVID vaccine between 12/2020 and 04/2021. The primary endpoint was a positive anti-COVID spike protein antibody titer following 2 doses of the COVID mRNA vaccines or 1 dose of the COVID adenovirus vaccine. Additional outcomes of interest included key variables, such as lymphoma subtype and treatment with anti-CD20 monoclonal antibodies. Subgroups were compared using Fisher's exact test, and unadjusted and adjusted logistic regression models were used for univariate (UVA) and multivariate (MVA) analyses.
Results
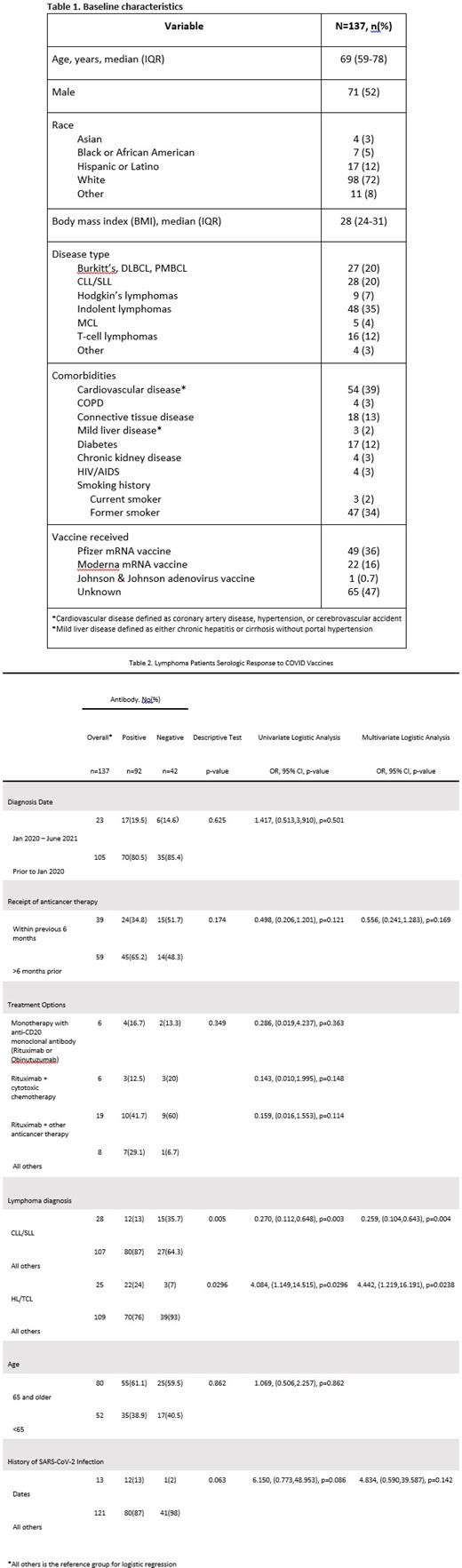
One-hundred thirty-seven patients were identified with baseline characteristics as shown in Table 1. Overall, the study population was older at a median age of 69 (IQR 59-78) years old, 52% of patients were male, and 72% of patients were white. The most frequent comorbidities were cardiovascular disease (39%) and former smoking history (34%), and 45 (33%) patients were obese (BMI >= 30).
Testing for anti-COVID spike protein antibodies occurred at a median 48 (IQR 25-62) days [range 6-120] after second vaccination. Lymphoma subtypes in our cohort were: indolent lymphomas (35%), CLL/SLL (20%), 27 (20%) patients with Burkitt's, DLBCL, PMBCL combined, and 25 (18%) patients with Hodgkin's and T-cell lymphomas (HL/TCL) combined. Majority of patients received COVID mRNA vaccines, and we were able to confirm the specific type in 71 (52%) patients. Only 1 person received the COVID adenovirus vaccine.
Ninety-two patients (67.2%) developed anti-COVID spike protein antibodies after receiving a COVID vaccine. Of 27 patients who received an anti-CD20 monoclonal antibody-containing regimen in the last 12 months prior to vaccination, 14 (52%) patients produced antibodies. This rate was numerically lower than 72% (26/36) of those who developed antibodies and received an anti-CD20 antibody greater than 12 months prior to vaccination.
There were differences observed in the ability to produce serology towards the COVID vaccine amongst lymphoma subtypes. Of 28 patients with CLL, 12 (43%) produced antibodies. There were 6 CLL patients receiving anticancer treatment at the time of vaccination, of which 2 patients produced antibodies. CLL/SLL patients were less likely to mount an antibody response to the COVID vaccine when compared to those with other types of lymphoma, and this difference was significant on UVA (OR 0.270, 95% CI 0.112-0.648), p=0.003) and MVA (OR 0.259, 95% CI 0.104-0.643, p=0.004). For patients with HL/TCL, 22 of 25 (88%) patients produced antibodies. Among the 3 HL/TCL patients that did not produce antibodies, 1 patient had HIV/AIDS post-transplant, 1 had relapsed AITL, and 1 received rituximab. All HL/TCL patients who received anticancer treatment in the last 6 months (10 of 10) produced antibodies at a median titer of 120 AU/mL (reference >=15 AU/mL), with 4 patients having a robust response of antibody titers >400 AU/mL. On statistical analysis, HL/TCL patients were more likely to elicit an antibody response to the COVID vaccine when compared to those with other types of lymphoma, and this response was significant on UVA (OR 4.084, 95% CI 1.149-14.515, p=0.03) and MVA (OR 4.442, 95% CI 1.219-16.191, p=0.024).
Conclusion
Lymphoma patients are capable of mounting a humoral response to the COVID mRNA vaccines. CLL/SLL appears predictive of a negative antibody response to the COVID vaccine, while HL/TCL histologies appeared to correlate to a positive antibody response, even with treatment within 6 months of vaccination. Our study suggests anti-CD20 monoclonal antibody therapy in the last 12 months may affect the ability to produce serology towards a COVID vaccine. Further studies are required to confirm our findings, including whether T-cell immunity would be of clinical relevance in this patient population.
1. Passamonti et al, Br J Haematol 2021
Leslie: Kite, a Gilead Company: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Speakers Bureau; PCYC/Janssen: Consultancy, Honoraria, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Seagen: Consultancy, Honoraria, Speakers Bureau; Epizyme: Consultancy, Honoraria, Speakers Bureau; Karyopharm Therapeutics: Honoraria, Speakers Bureau; Celgene/BMS: Consultancy, Honoraria, Speakers Bureau; Merck: Consultancy; Pharmacyclics: Consultancy, Honoraria, Speakers Bureau; ADC Therapeutics: Consultancy. Goy: Acerta: Consultancy, Research Funding; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Genentech/Hoffman la Roche: Research Funding; AbbVie/Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Vincerx pharma: Membership on an entity's Board of Directors or advisory committees; Rosewell Park: Consultancy; LLC(Targeted Oncology): Consultancy; Elsevier's Practice Update Oncology, Intellisphere, LLC(Targeted Oncology): Consultancy; Michael J Hennessey Associates INC: Consultancy; Hoffman la Roche: Consultancy; Xcenda: Consultancy; Medscape: Consultancy; Physicians' Education Resource: Consultancy, Other: Meeting/travel support; Vincerx: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie/Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria; MorphoSys: Honoraria, Other; Novartis: Consultancy, Honoraria; OncLive Peer Exchange: Honoraria; Xcenda: Consultancy, Honoraria; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Elsevier PracticeUpdate: Oncology: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Genomic Testing Cooperative: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; COTA (Cancer Outcome Tracking Analysis): Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership role; Hackensack Meridian Health, Regional Cancer Care Associates/OMI: Current Employment; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity/Verastem: Research Funding; Janssen: Research Funding; Karyopharm: Research Funding; Phamacyclics: Research Funding; Constellation: Research Funding. Feldman: Alexion, AstraZeneca Rare Disease: Honoraria, Other: Study investigator.