Abstract
Background
Myelofibrosis (MF) is the most aggressive subtype among classical BCR-ABL1 negative myeloproliferative neoplasms (MPN). Approximately 90% of patients harbor a mutation affecting JAK2, MPL, or CALR which results in constitutive activation of the JAK/STAT pathway, resulting in proliferative and dysfunctional blood cell production, extramedullary hematopoiesis, and constitutional symptoms. Remaining patients are deemed to be "triple-negative" (TN), a designation associated with a poor prognosis.
Methods
We identified patients with confirmed MF (inclusive of primary MF and MF occurring after essential thrombocythemia or polycythemia vera) treated at Moffitt Cancer Center between 2003-2021. Patients were deemed to be TN if they had tested negative for mutations involving JAK2, MPL and CALR. TN patients were compared to non-TN patients who exhibited a mutation in at least one of these genes. Patients with incompletely driver mutation testing were excluded. Baseline demographic, clinical and molecular characteristics were assessed. Kaplan-Meier method was used to determine overall survival (OS) and leukemia-free survival (LFS).
Results
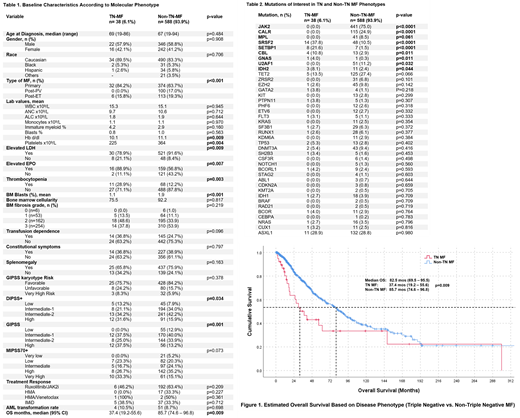
626 patients with a diagnosis of MF were identified, among which 6% (n=38) were confirmed to harbor TN disease. See Table 1 for baseline characteristics of TN vs non-TN patients.
Thrombocytopenia was more common in TN disease (28.9% vs 12.2%, p=0.003), as well as elevated EPO titers at baseline (88.9% vs 56.8%, p=0.007). Elevated LDH titers at baseline were less common with TN disease (78% vs 91%, p=0.009). Baseline Hb (p=.009), and % of marrow myeloblasts (p < .005) were lower in the TN cohort. Clinically, there were no differences regarding transfusion dependence, presence of constitutional symptoms or splenomegaly (Table 1).
Regarding prognostic scores, patients with TN MF exhibited higher-risk disease per DIPSS+ (65.8% vs 58.1%, p=.034) and GIPSS (62.5% vs 47.1%, p=.001) compared to their non-TN counterparts. There were no significant differences in IPSS, DIPSS+, MIPSS70 or MIPSS70+ stratifications.
The median OS (mOS) for the entire population was 82.5 months (95%CI 69.4-95.5). Patients with TN MF had shorter survival rates with a mOS of 37.4 months (95%CI 19.2-55.5) compared to 85.7 mo (95% CI 74.6-96.85) for non-TN disease (p=.009). The rate of transformation to AML was 10.5% for TN MF, 9.7% for JAK2, 7.3% for MPL and 5.2% for CALR MF (TN vs non-TN MF p=0.7). Median LFS was 65.2 mo for CALR, 34.1 mo for TN, 21.9 for JAK2 and 16.9 mo for MPL mutant MF (p = 0.498 for TN vs non-TN phenotypes).
Nominally, TN patients had fewer responses (46.2% vs 63.4%) and shorter duration of response to ruxolitinib (8.0 mo vs 12.5 mo), though this did not meet significance (p = 0.21 and 0.5, respectively). There were no differences in response rate to lenalidomide/thalidomide, HMA, HMA/venetoclax (Table 1)
Mutations involving SRSF2, SETBP1, IDH2, CBL, and GNAS were significantly enriched in TN disease (see table 2). U2AF1 mutations were more frequently seen in the non-TN cohort (11.2% vs 0%, p=0.032) (Table 2).
Conclusion
In our independent database of MF, we confirmed the unfavorable prognosis of TN-MF in terms of shorter OS and LFS. While lacking classic driver mutations, TN-MF frequently harbors mutations impacting splicing, epigenetic modification, and signaling that likely drive this aggressive clinical course, and may account for suboptimal responses to JAK inhibition.
Tinsley-Vance: Taiho: Consultancy; Jazz: Consultancy, Speakers Bureau; Astellas: Speakers Bureau; Fresenius Kabi: Consultancy; Abbvie: Honoraria; Novartis: Consultancy; Celgene/BMS: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau. Sallman: Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Intellia: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; Kite: Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees. Sweet: Gilead: Membership on an entity's Board of Directors or advisory committees; AROG: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet: ElevateBio Management: Consultancy; Millenium Pharma/Takeda: Consultancy; Celgene/BMS: Consultancy; Agios: Consultancy; Daiichi Sankyo: Consultancy; AbbVie: Consultancy; BerGenBio: Consultancy; Jazz: Consultancy; Astellas: Consultancy. Padron: Stemline: Honoraria; Taiho: Honoraria; BMS: Research Funding; Blueprint: Honoraria; Incyte: Research Funding; Kura: Research Funding. Kuykendall: Prelude: Research Funding; PharmaEssentia: Honoraria; Novartis: Honoraria, Speakers Bureau; Incyte: Consultancy; CTI Biopharma: Honoraria; Celgene/BMS: Honoraria, Speakers Bureau; BluePrint Medicines: Honoraria, Speakers Bureau; Abbvie: Honoraria; Protagonist: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Komrokji: AbbVie: Consultancy; Geron: Consultancy; PharmaEssentia: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Acceleron: Consultancy; Taiho Oncology: Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Speakers Bureau; BMSCelgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract