Abstract
Chimeric antigen receptor (CAR) T-cell therapy has proven highly effective in patients with hematological malignancies. However, resistance to CAR-T cell therapy arising from target protein shedding and other forms of antigen downregulation can lead to CAR-resistant disease relapse. Tumor escape may be successfully prevented through the simultaneous targeting of multiple tumor antigens. The ability to target multiple antigens with a single therapeutic modality offers the potential for anti-tumor responses, broader coverage of heterogeneous tumor populations, and the potential to prevent antigen escape, potentially inducing durable clinical remission. Multiple myeloma (MM) presents an ideal case to employ a dual-CAR approach, as BCMA-targeting cell therapies have shown impressive efficacy to date, but curative treatment remains elusive. Additionally, the oligoclonal nature of MM may contribute to antigen escape and clonal resistance. Here, we demonstrate the application of a unique dual-CAR approach simultaneously targeting two tumor associated antigens (TAA) for the treatment of MM. We further demonstrate the efficacy in an induced pluripotent stem cell (iPSC) platform, where a master engineered iPSC line is used as the starting material for mass production of off-the-shelf, dual-CAR immune effector cells.
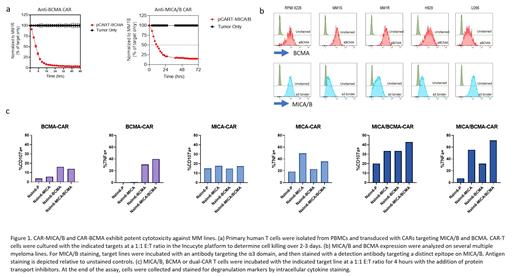
We selected B cell maturation antigen (BCMA), a well-defined TAA in MM, as the first antigen target. To develop the CAR-BCMA motif, we utilized our previously published high-affinity binding sequence shown to exhibit high selectivity to BCMA with enhanced recognition of low-BCMA expressing myeloma cells (Bluhm et al., Molec Ther 2018). As shown previously, the designed CAR-BCMA demonstrates potent and selective cellular cytotoxicity against MM (Figure 1a, left panel). BCMA has been observed to be actively cleaved from the surface of MM cells though, resulting in reduced efficacy and clinical relapse. To circumvent BCMA antigen escape, we developed a companion CAR targeting the pan-TAAs, MICA and MICB, which are expressed on MM plasma cells as well as monoclonal gammopathy of undetermined significance (MGUS) plasma cells. The CAR binding sequence targets the conserved α3 domain of MICA/MICB, which we have previously shown to inhibit MICA/B shedding and drive anti-tumor immunity (Andrade et al., Science 2018). The designed anti-MICA/B-α3 CAR exhibits selective targeting potential against an array of cancers, including the MM.1S cancer cell line (Figure 1a, right panel). To determine the suitability of co-targeting BCMA and MICA/B in MM, we surveyed surface expression patterns of BCMA and MICA/B antigens on a variety of MM cancer cell lines and observed a complimentary pattern of co-expression compatible with a dual-CAR to broaden targeting approach of malignant plasma cells (Figure 1b). Initial studies to evaluate the dual CAR approach in MM were performed by generating anti-BCMA and anti-MICA/B-α3 dual-CAR (MM dual-CAR) T-cells. MM dual-CAR T cells showed antigen-specific activation, degranulation and cytotoxicity against both antigens in an additive manner, consistent with the initial antibody staining on target cells and illustrating that co-targeting MICA/B and BCMA may increase the activity against MM (Figure 1c). Similar trends were observed in a series of cytotoxicity assays against several MM lines. Preliminary studies are ongoing in induced pluripotent stem cell (iPSC)-derived NK (iNK) cells expressing MM dual-CARs as a unique off-the-shelf cell therapy targeting both BCMA and MICA/B. Since MM dual-CAR iNK cells also express CD16, which mediates antibody-dependent cellular cytotoxicity, combination with therapeutic antibodies, such as anti-CD38 antibodies, can be deployed to target three TAAs for a complete therapeutic approach in MM. The data highlights the applicability of a multi-targeted approach in MM patients, whereby MM dual-CAR NK and/or T cells maintain responsiveness to malignant cells that shed or downregulate tumor antigens to evade treatment.
Lee: Fate Therapeutics, Inc.: Current Employment. Wucherpfennig: Novartis: Research Funding; SQZ Biotech: Membership on an entity's Board of Directors or advisory committees; TScan Therapeutics: Membership on an entity's Board of Directors or advisory committees; Immunitas Therapeutics: Current holder of individual stocks in a privately-held company; Nextechinvest: Membership on an entity's Board of Directors or advisory committees; TCR2 Therapeutics: Membership on an entity's Board of Directors or advisory committees. Bjordahl: Fate Therapeutics: Current Employment. Valamehr: Fate Therapeutics, Inc.: Current Employment.