Abstract
Background: The inherited bleeding disorder (IBD) community has witnessed significant advances in care, yet important gaps persist, particularly in rare disorders and underserved populations. An initiative spearheaded by the National Hemophilia Foundation (NHF) and shaped by the patient community aims to accelerate progress through a national research blueprint. The blueprint is being designed to identify and guide research priorities toward those areas that most significantly impact the lives of individuals affected today and articulate clearly defined opportunities to make the greatest impact for the future.
Methods: NHF has enlisted individuals with IBDs as subject matter experts (SMEs) to guide this initiative by elevating the most pressing issues affecting them today and informing expert discussions on actionable research priorities for the future. SME insights have been collected through listening sessions, a cross-community survey, and participation in multi-disciplinary working groups.
The NHF State of the Science Research Summit (SOS) in September 2021 will address the input of the working groups and will also feature patient vignettes to illustrate today's unmet needs and contextualize the research priorities identified to address them. As part of this interactive Summit, SMEs from traditionally underrepresented patient populations are also being enlisted to participate in remote participation groups (RPGs) with the goal of soliciting input that further tailors the research priorities to the needs of these populations. The RPGs will be comprised of individuals with bleeding disorders or their caregivers who represent specific populations by race/ethnicity (for example, African Americans, Asian Americans, Indigenous persons, Mexican and Central American Hispanic individuals, etc.). Within each group, NHF will aim to include individuals with diverse experiences based on their IBD, barriers related to access to care, gender and sexual orientation.
During each session, the moderated RPGs will participate in the live summit, discuss the expert dialogue, and share real-time perspectives and comments about how the content addresses, or not, their specific community needs. The expert SOS panel will then have the chance to address their comments. The commentary from these sessions will be included in the NHF blueprint to define the research path forward for the community. The RPGs are also expected to be reconvened in Spring 2022 to review and improve upon the opportunities identified in the blueprint.
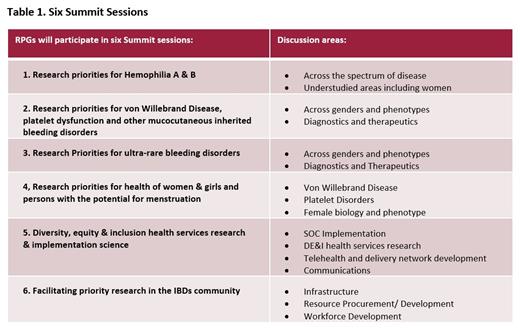
Results: NHF has enlisted broad and diverse community support to ensure the blueprint accurately represents the opportunities to create meaningful and lasting impact for individuals with IBDs. In total, 42 patients and caregivers participated in listening sessions; 125 contributed to the community survey; 15 are participating in the Summit working groups and approximately 200 are being enlisted for the remote participation groups. The themes to be addressed during the SOS reflect the input provided by the SMEs and health professionals (see Table 1).
Conclusions: Actively soliciting the patient community's views is central in our process to advance research in IBDs. By enlisting the participation of historically underserved community segments, this effort aims to address some of the most persistent and pressing issues affecting the IBD community today. Specific insights from the RPG participation in the Summit will be included in the presentation. This blueprint, which will guide the U.S. research community, could help fundamentally redefine the experience of diverse populations living with these disorders.
Witkop: Teralmmune, Inc.: Consultancy. Recht: Octapharma: Consultancy; Novo Nordisk: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; Takeda: Consultancy; uniQure: Consultancy; Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders: Speakers Bureau; American Thrombosis and Hemostasis Network: Current Employment; Oregon Health & Science University: Current Employment; Kedrion: Consultancy; Hema Biologics: Consultancy; Genentech: Consultancy; CSL Behring: Consultancy; Catalyst Biosciences: Consultancy. Valentino: Spark: Ended employment in the past 24 months.