Abstract
Background: Acute myeloid leukemia (AML) requires timely access to treatment for best patient outcomes. Despite available treatment options, including chemotherapy or allogeneic hematopoietic cell transplantation (alloHCT), some older patients do not receive any active therapy, particularly those aged 65-74. Although previous studies have analyzed costs and resource use associated with AML, little is known about the use of specific services by treatment approach, like HLA typing, palliative or hospice care, clinical trial participation, or cancellation of alloHCT within the first year of AML diagnosis. This study aimed to identify and analyze service utilization of specified services for patients age 65-74 diagnosed with AML on Medicare, by treatment approach (chemotherapy alone [chemo], chemotherapy and alloHCT [alloHCT], and no chemotherapy or alloHCT [no chemo]).
Methods: Administrative claims data from the Center for Medicare and Medicaid Services (CMS) from 2010 to 2016 were used for analysis. Patients aged 65-74 diagnosed with AML between 2010 and 2015 and enrolled in fee-for-service (Part A and B) Medicare for at least one year after diagnosis or until death, were included. Chemo, alloHCT and specified services (HLA typing, palliative or hospice care, and clinical trial participation) or HCT cancellation were identified using diagnosis and procedure codes; at least one claim for the specified service had to be present in the first year after diagnosis. Overall survival (OS) within one year of diagnosis by treatment approach was calculated using Kaplan-Meier survival curves.
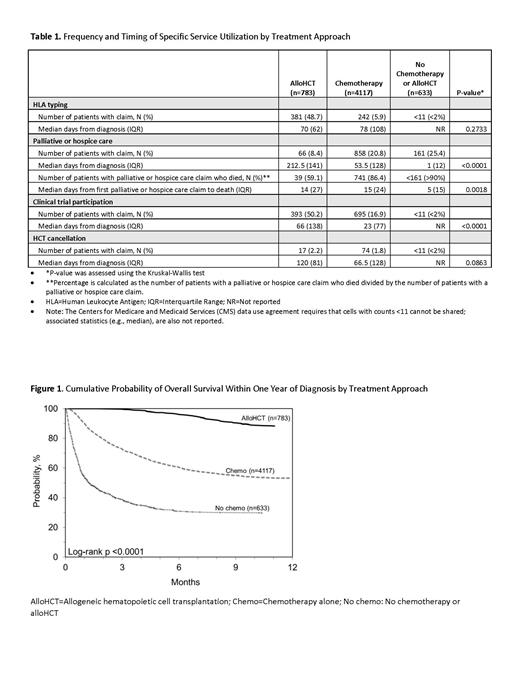
Results: A total of 5533 patients met eligibility criteria: 783 (14.1%) received alloHCT; 4117 (74.4%) received chemo; and 633 (11.4%) received no chemo within the first year of diagnosis. Patients in all treatment groups had an HLA-typing claim: 48.7% in the alloHCT cohort, 5.9% in the chemo alone cohort, and <2% in the no chemo cohort. 8.4% of patients receiving alloHCT had a claim for palliative or hospice care, 20.8% in the chemo cohort and 25.4% in the no chemo cohort. Clinical trial participation claims occurred in 50.2% of alloHCT patients, 16.9% of chemo patients, and <2% of the no chemo patients. HCT cancellation claims occurred in 2% or less of beneficiaries in each treatment approach. Time from diagnosis to palliative or hospice care and to clinical trial participation were statistically significantly different among the three treatment approaches (Table 1). Time from first palliative or hospice care claim to death was also significantly different across the treatment groups. There was a significant difference in one-year OS across the three treatment groups (p<0.0001); the alloHCT cohort had the highest one-year OS (Figure 1).
Conclusions: This study used a population-based approach to identify specific service utilization by treatment approach. Due to the fast progression of AML, timely receipt of treatment and services is critical. Patients in all three treatment groups had claims for HLA typing, palliative or hospice care, HCT cancellation, and clinical trial participation. Palliative or hospice care occurred most frequently in the no chemo cohort and occurred more closely to diagnosis, compared to the alloHCT and chemo cohorts. Clinical trial participation occurred most commonly in the alloHCT cohort, though occurred sooner in the chemo cohort.
Claims data is collected for billing rather than research and has some inherent limitations; treatment approach was defined using the presence of codes in the data, not on disease severity and cytogenetics, which is not available in the claims data. Specified service utilization might be underestimated due to missing information or coding errors. Additionally, the potential for bundling of services in claims may limit the ability to identify specific services and may undercount these services, illustrated by the fact that less than 50% of the alloHCT group had a claim for HLA typing. Despite these limitations, claims data is an important source of resource and service utilization.
Further analysis will be done to study referral patterns upon diagnosis, to determine predictors of service utilization and timing of care and to expand the timeframe of observation beyond one-year. Study results will be used to help inform strategies to improve timely access to services and treatment to help improve care, and ultimately outcomes, for patients diagnosed with AML.
No relevant conflicts of interest to declare.