Abstract
Introduction: Daratumumab, a CD38 monoclonal antibody, was approved for the treatment of heavily pretreated relapsed or refractory multiple myeloma (MM) in 2015 and for newly diagnosed MM (NDMM) in 2018. The phase 3 MAIA study demonstrated that daratumumab plus lenalidomide/dexamethasone (D-Rd) improved clinical outcomes, including overall survival, versus lenalidomide/dexamethasone (Rd) in transplant-ineligible NDMM (Facon T, et al. EHA Library. 2021). However, limited real-world data are available regarding patients with MM treated with daratumumab in the US. We evaluated patient characteristics and treatment outcomes among transplant-ineligible NDMM patients who received D-Rd as first-line therapy in US oncology practices.
Methods: This retrospective, observational cohort study evaluated patients from the Flatiron MM Core Registry who received first-line D-Rd between November 1, 2015 and February 28, 2021. The Flatiron Health electronic health record (EHR)-derived deidentified database has longitudinal patient-level records for patients treated at community oncology practices and academic medical centers across the US. The index date was the date of the first observed record of the D-Rd regimen. Patients aged <18 years on the index date, enrolled in a clinical trial on the index date, with other malignancies prior to the index date, with a diagnosis of amyloid light-chain amyloidosis prior to the index date, or with hematopoietic stem cell transplant from the diagnosis date to the index date were excluded. Patient characteristics analyzed included age at time of D-Rd initiation, race, Eastern Cooperative Oncology Group performance status (ECOG PS) score, International Staging System (ISS) stage, frailty, and presence of comorbidities such as diabetes and acute renal impairment. Time-to-next-treatment and progression-free survival (PFS) were analyzed using the Kaplan-Meier method.
Results: From November 1, 2015 to February 28, 2021, 1,721 patients were identified from the Flatiron MM Core Registry as patients treated with a daratumumab-based regimen; 120 (7.0%) of the 1,721 patients were treated with a daratumumab-based regimen as first-line therapy, and 35 (29.2%) of the 120 patients were identified as eligible patients treated with D-Rd as first-line therapy. For patients treated with first-line D-Rd (n=35), the mean age as of the index date was 73.5 years (SD: 7.9). Most patients were White (72.4%), had an ECOG PS score of 1 (51.9%), and had an ISS stage of II (38.1%) or III (42.9%). Of these patients, 15 (42.9%) patients were ≥75 years of age, 4 (11.4%) were Black or African American, 4 (11.4%) had high-risk cytogenetics, 21 (60.0%) were frail, 6 (17.1%) had diabetes, and 6 (17.1%) had acute renal impairment.
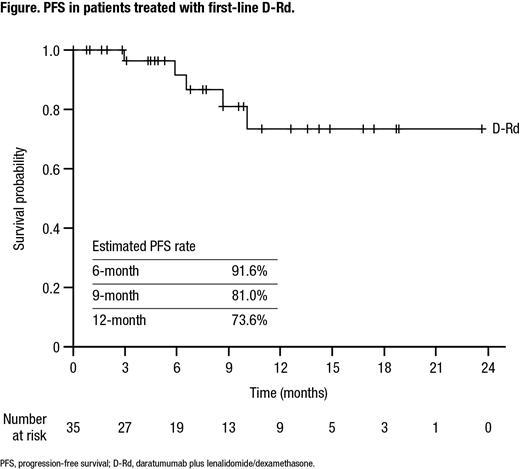
The median time from the initial diagnosis date to the index date was 1.2 months and the median follow-up was 8.0 months. The median PFS was not reached in this study. The estimated 6-month PFS rate was 91.6%, 9-month PFS rate was 81.0%, and 12-month PFS rate was 73.6% (Figure). After 15 months of follow-up, an estimated 80.5% of patients had not received their next treatment.
Conclusions: The real-world population of patients who received D-Rd as first-line therapy is similar to the population studied in MAIA. The early trend of PFS is also similar to that in MAIA, with the majority of patients progression free at 6, 9, and 12 months. Greater understanding of long-term outcomes among transplant-ineligible NDMM patients who received D-Rd as first-line therapy at US oncology practices is needed to further facilitate the safe and effective use of daratumumab.
Tai: Janssen Scientific Affairs, LLC: Current Employment. Cai: Janssen: Current Employment. Fu: Janssen: Current Employment. Khare: Janssen: Current Employment. Kaila: Janssen Scientific Affairs, LLC: Current Employment.