Abstract
Background:
Several observational studies have reported the rate of thrombotic events in patients infected with coronavirus disease 2019 (COVID-19), with conflicting results 1. The conflicting results could be partially explained by different population critically ill vs non critically ill, different definition of thrombotic events, follow up period was variable, and different prophylaxis that were used. Middle east respiratory syndrome (MERS-COV) is another coronavirus had been initially reported in Saudi Arabia in 2012. A genome scan has shown a 50% similarity between COVID-19 and MERS-COV 2.A common features of covid-19 and MERS-COV including transmissibility, and MERS-COV clinical presentation have been identified 3. However, data about thrombotic complications in patients with MERS-COV are limited.
The aim of this study was to compare the rate of thrombotic events between patients with COVID-19 and MERS-COV.
Methods:
Patients :
We included all confirmed COVID-19 patients who were admitted to intensive care unit (ICU) in 3 major hospitals in Saudi Arabia between February and July 2020. We included all confirmed cases of MERS-COV who were admitted to ICU from these centers between March to May 2014. Patients were excluded if they were transferred in or out from one of these three hospital to another hospitals. Data were collected retrospectively from the first day of admission until discharge or death.
Outcome:
The primary outcome was the rate of venous thromboembolism (VTE). The secondary outcomes were the rate of arterial events, the rate of composite events (venous and arterial) and the rate of bleeding. VTE included all symptomatic or incidentally diagnosed cases of pulmonary embolism (PE), deep vein thrombosis (DVT) and thrombosis in unusual sites (cerebral, mesenteric, portal, splenic, hepatic, and renal veins). All VTEs were confirmed radiographically by appropriate imaging. Screening for VTE in asymptomatic patients was not performed. If more than on type of VTE occurred in the same patient, it was considered one event. Arterial events included cerebrovascular accidents (CVAs), mesenteric ischemia, and limb ischemia and were confirmed by the appropriate imaging modality. Myocardial infarction (MI) was diagnosed based on the suspicion of the attending physician using clinical criteria as well as biomarker elevations or electrocardiographic changes. Composite events were defined as any VTE or arterial event. Bleeding events were classified as major and nonmajor based on the definition of international society of thrombosis and hemostasis (ISTH) 4. Informed consent was waived.
Statistical analysis:
Characteristics and outcomes were compared between COVID-19 and MERS-COV groups using chi-square test, fisher exact test or t-test. The rates of thrombosis and bleeding are summarized as proportions, with the corresponding 95 % confidence intervals (CI). A P-value less than 0.05 was considered significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA)
Result:
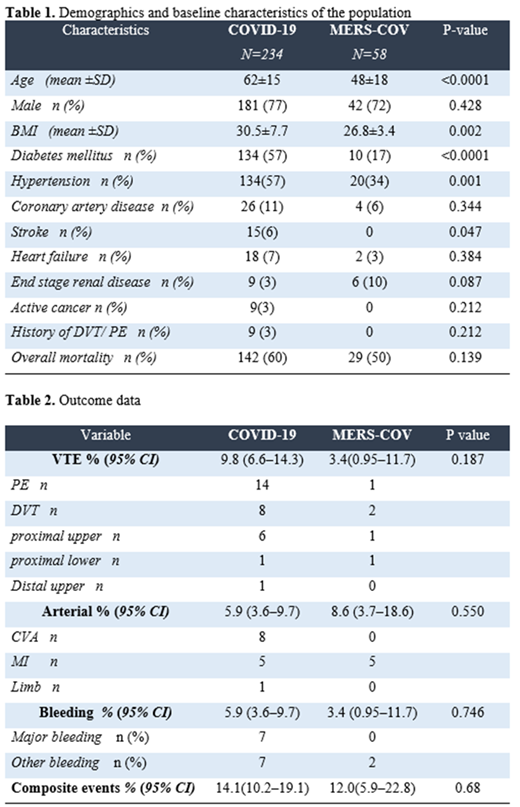
After exclusion, 234 COVID-19 and 58 MERS-COV patients were included. The majority of patients with COVID-19 (98%, n=230) and more than (67% ,n =39 ) of those with MERS-COV group received pharmacological prophylaxis. The most frequently prescribed regimen in both groups was enoxaparin (40 mg twice per day). Over a median length of stay in the COVID-19 group of 22 days, the rate of VTE 9.8%(CI; 6.6-14.3) and was 3.4%(CI; 0.95-11.7) in the MESR-COV group over median length of stay of 10 days. The rate of arterial events were 5.9 % (CI;3.6-9.7) and 8.6% (CI;3.7-18.6) in COVID-19 and MERS-COV respectively. Table 1 and 2.
Conclusions:
To our knowledge, this is the first study compared thrombotic risk between COVID-19 and MERS-COV. We found a similar rate of composite thrombotic events (venous and arterial ) between COVID-19 and MERS-COV with higher rate of venous thrombosis in COVID-19 and higher rate arterial thrombosis in MERS-COV. This may indicate that not only COVID-19 is a prothrombotic disease, but MERS-COV may have similar risk of thrombotic complication . These result needs to be confirmed in a larger studies.
References
1- Al-Samkari, H, 2020. Blood, 136(4), Pp.489-500.
2- Lu, R, 2020. The Lancet, 395(10224), Pp.565-574.
3- Petrosillo, N, 2020. Clinical Microbiology And Infection, 26(6), Pp.729-734.
4- Schulman, S, 2005. Journal Of Thrombosis And Haemostasis, 3(4), Pp.692-694.
No relevant conflicts of interest to declare.