Abstract
Introduction
The COVID-19 pandemic disrupted non-urgent and preventive medical care. During the early peak of the pandemic, an estimated 41% of US adults delayed or avoided medical care (Czeisler et al, CDC, 2020). While there were documented declines in the number of emergency department visits for myocardial infarction, stroke and hyperglycemia, similar data is not available related to acute myeloid leukemia (AML) (Lange et al, CDC, 2020). A delay in the diagnosis of AML could lead to presentation when patients are less able to withstand chemotherapy or have a higher disease burden which could compromise overall survival (OS). In this retrospective analysis, we aim to elucidate if there was a difference in clinical, cytogenetic, or molecular presentations and if there was an effect on early mortality as determined by overall survival at 1 and 6 months.
Methods
We compared the clinical, cytogenetic, and baseline molecular genetics of consecutive adult patients diagnosed with de novo AML at Dana-Farber Cancer Institute/Brigham and Women's (DFCI/BWH) Hospital from March 23, 2020, the date of the Massachusetts COVID State of Emergency, to August 23, 2020 to a historical cohort of similar patients between presenting between March 23, 2017 and August 23, 2020. Data was obtained from the Hematological Malignancy Data Repository and via review of the medical record. Patients were excluded from this cohort if they were diagnosed with acute promyelocytic leukemia, had known antecedent myeloid malignancy, or if they did not have DFCI/BWH 96-gene next-generation sequencing panel (RHP) performed at the time of diagnosis.
Baseline clinical, laboratory, cytogenetic, and molecular characteristics and outcomes were compared between the pre-pandemic and pandemic cohorts using chi-squared, Fisher's exact, and Wilcoxon rank sum analyses (where appropriate) at a significance of p<0.05.
Results
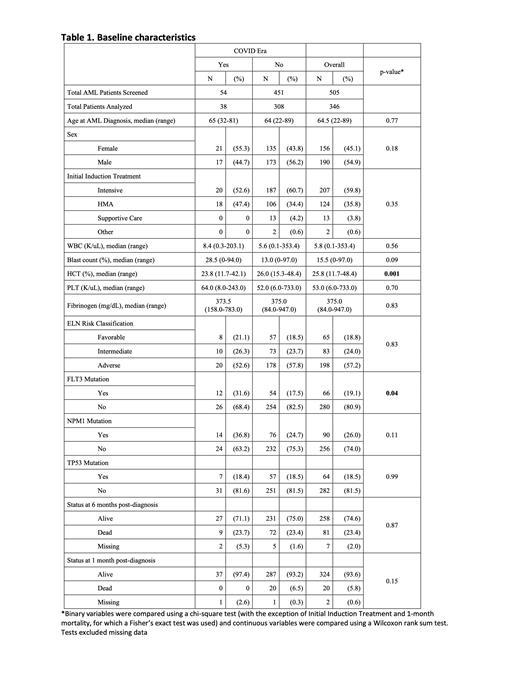
Thirty-eight AML patients presented during the COVID-19 pandemic (PAN) and 308 in the pre-pandemic (PREPAN) period. There was no statistically significant difference in the monthly rate of new patients presenting in PREPAN and PAN cohorts (8 vs. 6 new patients/month, p=0.73). The median age at presentation (64 PREPAN vs. 65 PAN, p=0.77), sex, and therapeutic approach (intensive, non-intensive, supportive care, other) were not statistically different between cohorts. Presenting white blood cell count, platelet count, and fibrinogen were not different between cohorts, while hematocrit was significantly lower in the PAN cohort (23.8% vs. 26.0%, p=0.001). There was a trend for a higher median blast percentage (28.5% vs. 13%, p=0.09) in the PAN cohort.
There were no differences between the cohorts in the median number of cytogenetic abnormalities, nor in the incidence of complex karyotype, (25.3% vs. 23.7%) across PREPAN and PAN respectively. There were also no significant differences in the European LeukemiaNet (ELN) risk classification scores across the PREPAN and PAN time periods, with 57.8% vs. 52.6% of total patients presenting with adverse risk disease respectively. When specific mutations of TP53, NPM1, and FLT3 were evaluated, only FLT3 demonstrated a statistical difference with a higher proportion in the pandemic group (p=0.04).
OS at 1-month (97.4% and 93.2%, p=0.15) and 6-months (71.1% and 75.0%, p-0.87) were not statistically different in the PREPAN and PAN cohorts, respectively.
Conclusion
These data represent a novel analysis of the presenting clinical, cytogenetic and molecular characteristics of de novo AML during the COVID-19 pandemic. In contrast to other diseases, we did not see fewer de novo AML presentations during the peak of the COVID pandemic. While the reasons are unknown and require validation in large cohorts, the symptoms of leukemia including symptomatic anemia (low hematocrit) and higher WBC and blast count possibly driven by FLT3 mutations may drive patients to seek emergent clinical evaluation despite COVID pandemic barriers.
The lack of difference in cytogenetic or other prognostic entities may demonstrate a lack of symptom correlation causing patients to present for care. The higher incidence of FLT3 mutations and lower hematocrit could reflect more symptomatic presentation of AML during the COVID pandemic. Since these differences may be a surrogate for a higher disease burden, it will be important to compare outcomes at longer time points.
DeAngelo: Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Jazz: Consultancy; Incyte: Consultancy; Forty-Seven: Consultancy; Autolus: Consultancy; Amgen: Consultancy; Agios: Consultancy; Takeda: Consultancy; Glycomimetrics: Research Funding; Blueprint: Research Funding; Abbvie: Research Funding; Servier: Consultancy. Stone: Bristol Meyers Squibb: Consultancy; Astellas: Membership on an entity's Board of Directors or advisory committees; BerGen Bio: Membership on an entity's Board of Directors or advisory committees; Boston Pharmaceuticals: Consultancy; Innate: Consultancy; Foghorn Therapeutics: Consultancy; Gemoab: Membership on an entity's Board of Directors or advisory committees; Glaxo Smith Kline: Consultancy; Celgene: Consultancy; Elevate Bio: Membership on an entity's Board of Directors or advisory committees; OncoNova: Consultancy; Syntrix/ACI: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Agios: Consultancy, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Aprea: Consultancy; Arog: Consultancy, Research Funding; Jazz: Consultancy; Macrogenics: Consultancy; Novartis: Consultancy, Research Funding; Actinium: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Syros: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy. Garcia: AstraZeneca: Research Funding; Prelude: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Winer: Abbvie: Consultancy; Takeda: Consultancy; Novartis: Consultancy.