Abstract
Background: Polycythemia vera (PV) is a myeloproliferative neoplasm characterized by clonal proliferation of hematopoietic progenitor cells leading to elevated peripheral blood counts. Patients with PV have an increased risk of thrombotic events (TEs). Advanced age and a history of TEs are the components of the conventional risk model used to determine thrombosis risk and treatment strategy (Marchioli. J Clin Oncol. 2005;23:2224-32). Although, multiple studies have shown an association between TEs and elevated hematocrit (HCT) levels, the association between TEs and white blood cell (WBC) or platelet (PLT) counts has not been assessed in a consistent manner. The large, real-world, p rospective Obs ervational Study of Patients with Polycythemia Vera in US Clinic al Practices (REVEAL; NCT02252159) followed patients with PV treated in both community and academic practices. This analysis evaluated the association between elevated blood counts and occurrence of TEs in patients with PV using data from the REVEAL study.
Methods: Patients were required to have ≥3 lab values (blood counts) in the post-enrollment period and were excluded if they had a post-enrollment TE but did not have a lab value <6 months before that TE. The association between blood count status and TEs was assessed using a time-dependent covariate Cox proportional hazards model. Time to first post-enrollment TE was modeled with time censored at last known visit for patients without a TE. Each lab parameter was individually modeled with sex, age, disease duration and TE history at enrollment included as baseline covariates and treatment (hydroxyurea, other PV treatment) as a time-dependent covariate. Blood counts were included as binary time dependent covariates using the following thresholds: HCT >45%, WBC >11×10 9/L, PLT >400×10 9/L (Barosi G. Blood. 2013;121:4778-81; Barbui T. Blood. 2015;126:560-1). Linear interpolation was used to determine lab values between observed lab values. Alternative thresholds for WBC (<7, ≥7 to <8.5; ≥8.5 to <11, and ≥11×10 9/L [Barbui T. Blood. 2015;126:560-1]) and PLT counts (>600×10 9/L [Barosi G. Blood. 2009;113:4829-33; Regev A. Am J Hematol. 1997;56:168-72]) were evaluated. P values <0.05 were considered statistically significant.
Results: Of 2510 patients enrolled, 2271 were eligible for this analysis (77.9% high- and 22.1% low-risk). The median age was 66 years (range, 22-95), and 1229 (54.1%) were male. The median disease duration was 4.1 years (range, 0-56.3), 456 (20.1%) had a TE history, and most patients (52.6%) were receiving hydroxyurea. Out of 106 patients who had TEs, 30 had arterial TEs (most commonly, transient ischemic attack [n=15]) and 76 had venous TEs (most commonly, deep vein thrombosis [n=37]).
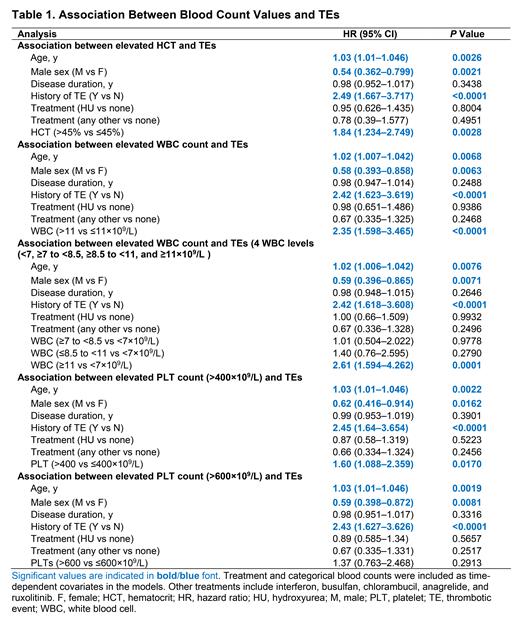
Elevated HCT levels (>45%, hazard ratio [HR]=1.84 [95% CI, 1.234-2.749], P=0.0028), WBC (>11×10 9/L, HR=2.35 [1.598-3.465], P<0.0001), and PLT counts (>400×10 9/L, HR=1.60 [1.088-2.359], P=0.0170) were each associated with increased TE risk (Table 1). Compared with alternative thresholds, WBC count ≥11×10 9/L is associated with the highest TE risk compared with WBC count <7×10 9/L (HR=2.61 [95% CI, 1.594-4.262], P<0.0001). An alternative threshold for PLT count (>600×10 9/L) was indicative of increased TE risk (HR=1.37 [95% CI, 0.763-2.468]) compared with PLT count ≤600×10 9/L, but this was not statistically significant (P>0.05). In all models, advanced age, female sex, and TE history were associated with increased TE risk.
Conclusion: This analysis of REVEAL, the largest real-world cohort of PV patients to date, demonstrated that elevated HCT levels (>45%), as well as WBC (>11×10 9/L) and PLT counts (>400×10 9/L) were each associated with increased TE risk. These data support the need to incorporate blood count values into risk stratification and treatment strategies for patients with PV in clinical practice and to move beyond the conventional risk model. Further studies to understand the causal relationship between elevated blood counts and TEs are warranted. Additional analyses of REVEAL are ongoing to understand the relationship between TE risk and isolated WBC elevation, as well as time-dependent risk with elevated blood counts.
Gerds: Constellation: Consultancy; AbbVie: Consultancy; Brystol Myers Squibb: Consultancy; Sierra Oncology: Consultancy; Novartis: Consultancy; PharmaEssentia: Consultancy; Incyte: Research Funding; Constellation: Research Funding; Krtos: Research Funding; CTI Biopharma: Research Funding; Imago: Research Funding; Accutate: Research Funding. Mesa: Celgene: Research Funding; CTI: Research Funding; Samus: Research Funding; Abbvie: Research Funding; La Jolla Pharma: Consultancy; Promedior: Research Funding; Incyte Corporation: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; CTI: Research Funding; Constellation Pharmaceuticals: Consultancy, Research Funding; Novartis: Consultancy; Pharma: Consultancy; Gilead: Research Funding; AOP: Consultancy; Genentech: Research Funding. Burke: Bristol Myers Squibb: Consultancy; Roche/Genentech: Consultancy; Beigene: Consultancy, Speakers Bureau; Kymera: Consultancy; Verastem: Consultancy; SeaGen: Consultancy, Speakers Bureau; AbbVie: Consultancy; Epizyme: Consultancy; MorphoSys: Consultancy; X4 Pharmaceuticals: Consultancy; Kura: Consultancy; Adaptive Biotechnologies: Consultancy; AstraZeneca: Consultancy. Grunwald: Astellas: Consultancy; Daiichi Sankyo: Consultancy; Agios: Consultancy; Stemline: Consultancy; PRIME: Other; MDEdge: Other; Bristol Myers Squibb: Consultancy; Incyte: Consultancy, Research Funding; AbbVie: Consultancy; PER: Other; Janssen: Research Funding; Blueprint Medicines: Consultancy; Karius: Consultancy; Gilead: Consultancy; Amgen: Consultancy; Trovagene: Consultancy; Pfizer: Consultancy; Cardinal Health: Consultancy; Med Learning Group: Other; Sierra Oncology: Consultancy. Stein: Constellation Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pharmaessentia: Membership on an entity's Board of Directors or advisory committees. Scherber: Incyte Corporation: Current Employment, Current holder of stock options in a privately-held company. Yu: Incyte Corporation: Current Employment, Current holder of individual stocks in a privately-held company. Hamer-Maansson: Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Oh: Abbvie: Consultancy; Blueprint Medicines: Consultancy; Celgene/Bristol Myers Squibb: Consultancy; Constellation: Consultancy; CTI BioPharma: Consultancy; Disc Medicine: Consultancy; Geron: Consultancy; Incyte Corporation: Consultancy; Kartos Therapeutics: Consultancy; Novartis: Consultancy; PharmaEssentia: Consultancy; Sierra Oncology: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract