Abstract
Aim:
Primary Central Nervous System Lymphoma (PCNSL) [i.e. diffuse large B-cell lymphoma of the CNS] is a rare and poor-prognosis disease occurring predominantly in older patients (median age >60 years old). Prospective studies of two commonly used chemoimmunotherapy (CIT) protocols, MATRix and MPV/Ara-C (± rituximab), have reported 2-year PFS and OS of 57-61% and 69-81% respectively. Our aim was to evaluate registry-reported outcomes of frontline CIT strategies employed at Australasian sites.
Method:
A retrospective study of consecutive, immunocompetent, adult PCNSL patients (WHO criteria: 2017) treated with curative-intent CIT, from 10 sites (9 Australian, 1 Singaporean) between 1 st January 2009 and 31 st December 2018 (i.e. ten-year period). Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier (log-rank) method. Univariate associations were derived using a Cox model with variables p<0.10 entered stepwise into a multivariate model.
Results:
Data was collected on 207 patients, 189 of whom met WHO diagnostic criteria for PCNSL (i.e. diffuse large B-cell lymphoma of the CNS). We excluded patients with insufficient data (6), non-DLBCL histology (6), secondary PCNSL (3) and post-transplant lymphoproliferative disorder (3). Of these, 176 (93%) received curative-intent CIT. The majority (66%) were over 65 years of age (median: 65, range: 25-87); ECOG performance status was ≥ 2 in 31% (data not available for 14% of patients). The majority were male (55%) and had deep structure involvement (64%). International Extranodal Lymphoma Study Group (IELSG) risk criteria could not be calculated in many patients due to missing data (predominantly LDH and CSF protein). CSF involvement was rare (n=23, 13%) but data was only available for 60% of patients. Of the 159 with documented renal function, 26% had renal impairment (defined as Cockroft-Gault creatinine clearance <60ml/min or eGFR <90ml/min).
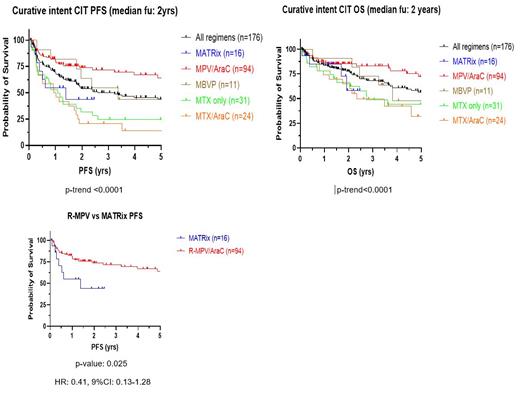
Five CIT regimens were used: MATRix (n=16), MPV/Ara-C + rituximab (Rtx) (n=94), MBVP+- Rtx (n=11), MTX +-Rtx (n=31) and MTX/Ara-C +- Rtx (n=24). Intrathecal chemotherapy was used in only 29 patients (16%), and almost exclusively in combination with R-MPV. Median cumulative MTX dose was 17,500mg/m 2 (range: 1,000-64,000mg/m 2) and 69% received Ara-C (median dose: 12,000mg/m 2 [range: 1,000-44,000mg/m 2]). Eighty percent of patients achieved an overall response at the end of MTX therapy, with 52% achieving a complete response [data unavailable in n=29, 16%]. Estimated 2-year PFS and OS for the entire cohort were 54% (95%CI: 0.46-0.62) and 77% (95%CI: 0.70-0.83) respectively at a median follow-up of 2 years. Older patients (>60yo) had shorter PFS but similar OS compared to their younger counterparts (2-year PFS: 47% vs 68%, p: 0.015, HR: 1.73, 95%CI: 1.11-2.70; 2-year OS: 74% vs 80%, p: 0.145, HR: 1.38, 95%CI: 0.81-2.33). R-MPV achieved superior PFS compared to MATRix although comparison was limited by low numbers in the MATRix cohort (n=16 vs n=94), likely reflective of the census period [2-year PFS: 74% vs 44% p: 0.025, HR 0.41, 95%CI 0.13-1.28]. Neither WBRT (n=57, 32%) nor ASCT (n=13, 7%) conferred a survival advantage but addition of rituximab (n=153, 87%) was associated with improved PFS (p: 0.007, HR: 0.47, 95%CI: 0.19-0.75). On multivariate analysis, type of induction CIT (p: 0.004, HR: 1.42, 95%CI: 1.23-1.42), cumulative MTX dose (p: 0.022, HR: 0.88, 95%CI: 0.83-0.94) and cumulative Ara-C dose (p: 0.016, HR: 0.76, 95%CI: 0.64-0.87) were associated with improved PFS. Cumulative MTX dose (p: 0.02, HR: 0.85, 95%CI: 0.78-0.92) and cumulative Ara-C dose (p: 0.007, HR: 0.67, 95%CI: 0.51-082) were also associated with improved OS.
Conclusion:
Registry-reported outcomes of contemporary CIT induction for PCNSL are favourable when compared to published trials and historical regimens. PCNSL with contemporary treatment should no longer be considered an invariably poor-prognostic disease. Data supports recent literature highlighting prognostic significance associated with maintenance of chemotherapy dose intensity (Martinez-Calle et al., Br J Haematol. 2020 Aug; 190(3):394-404. doi. 10.1111/bjh.16592).
Lewis: AstraZeneca: Consultancy, Honoraria; Janssen: Honoraria, Patents & Royalties: Conference attendance; Novartis: Patents & Royalties: Conference attendance; Roche: Consultancy, Honoraria. Gunjur: Myers Squibb: Honoraria. Ku: Roche: Consultancy; Antegene: Consultancy; Genor Biopharma: Consultancy. Wight: Jannsen: Honoraria, Other: Travel subsidies; Abbvie: Honoraria, Other: Travel subsidies. Shortt: Amgen: Research Funding; Astex: Research Funding; BMS: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees. Talaulikar: Roche: Honoraria, Research Funding; Jansenn: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; EUSA Pharma: Honoraria, Research Funding. Hawkes: Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accommodation expenses, Research Funding, Speakers Bureau; Regeneron: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Merck KgA: Research Funding; Bristol Myers Squib/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Specialised Therapeutics: Consultancy; Antigene: Membership on an entity's Board of Directors or advisory committees. Cheah: MSD: Consultancy, Honoraria, Other: advisory, Research Funding; Janssen: Consultancy, Honoraria, Other: advisory; TG Therapeutics: Consultancy, Honoraria, Other: advisory; Roche: Consultancy, Honoraria, Other: advisory and travel expenses, Research Funding; Loxo/Lilly: Consultancy, Honoraria, Other: advisory; AstraZeneca: Consultancy, Honoraria, Other: advisory; Celgene: Research Funding; AbbVie: Research Funding; Beigene: Consultancy, Honoraria, Other: advisory; Ascentage pharma: Consultancy, Honoraria, Other: advisory; Gilead: Consultancy, Honoraria, Other: advisory. Opat: Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Sandoz: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Monash Health: Current Employment. Gregory: Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel fees, Speakers Bureau; Janssen: Consultancy; Novartis: Consultancy.