Abstract
Introduction - primary refractory AML is associated with a dismal prognosis. Only 30% of patients will respond to salvage chemotherapy and continue to allogeneic hematopoietic cell transplantation (HCT) with a 10-20% long- term overall survival. Following from the FLAMSA protocol, we implemented a modified RIC regimen - FITCy (fludarabine, cytarabine +/- idarubicin and 4 Gy TBI) in all primary refractory patients with a donor available for transplant.
Methods - all patients who were admitted with AML, were identified by the transplantation coordinator nurse and underwent donor evaluation within 2 days from diagnosis. Patients who received induction chemotherapy had a day 14 marrow examination and where leukaemia blasts were identified, donor search was prioritized. These patients went on to have a day 28 bone marrow examination to confirm refractory disease. Patients who were treated with azacitidine ±venetoclax had a 2-month BM evaluation and donor search was prioritized in case of refractory disease. All consecutive patients, diagnosed with primary refractory AML, underwent HCT with the FITCy regimen in the Tel Aviv Sourasky Medical Center. Patients who underwent sequential therapy for relapsed disease (either chemosensitive or refractory) or a second HCT were excluded from this analysis. The protocol was amended on January 2018 to include ATG for all patients after interim analysis showed a high rate of GVHD.
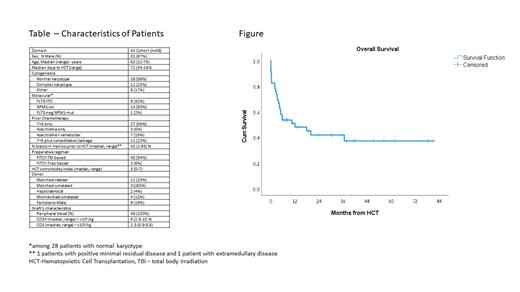
Results - Between January 2014 and June 2021, 48 patients were identified with primary refractory disease and were eligible for the protocol. Median age was 63 (range, 22-75) years (Table). Median follow-up for surviving patients was 33 (range, 1-81) months. Prior to HCT, 12 (25%) patients had ongoing documented infections (either microbiology or clinically). Median days to engraftment of neutrophils (>500/dL) and to complete engraftment of platelets (>20000/dL) were 10 (range, 7-20) days, and 16 (9-35) days, respectively. 7 patients with early progression/non relapse mortality were not evaluated for this outcome. No patient had primary/secondary graft rejection. Severe mucositis was observed in only 7 patients (15%) and was mostly observed in the upper gut. 18 patients (38%) developed microbiology documented infections during the neutropenic period and in 7 (40%) this directly contributed to mortality. Only 2 patients (4%) developed sinusoidal obstruction syndrome. Complete remission was documented on day 30, in all but 1 patient with a median whole marrow donor chimerism of 98% (range, 73-100%).Cumulative incidences of grade 2-4 and 3-4 acute GVHD at day 100 were 55% and 15%, respectively. Cumulative incidences of overall chronic GVHD and moderate-severe chronic GVHD at 1 year after HCT were 64% and 24%, respectively. Cumulative incidences of relapse at 1 year and 2 years post HCT were 24% and 30%, respectively. Non-relapse mortality rates, at 30 days, 100 days and 1 year post HCT were 13%, 20%, and 32%, respectively. Cumulative incidences of 1, 2, and 3 years overall survival were 50%, 42%, and 42%, Figure. Cox regression analysis for overall survival identified increased time from diagnosis (HR-1.03, p=.046), mismatched donor (HR-1.4, p=.05) and the use of ATG (HR=.33, p=.006) to impact survival, while age, sex and comorbidity index were not predictive.
Conclusions - Sequential therapy in patients with primary refractory AML provides a remarkable anti- leukemic effect. A timely donor search program is essential for the succession of this approach. Nevertheless, infection control and GVHD prophylaxis is still suboptimal and future protocols should focus on these domains while preserving graft vs. leukemia function.
Moshe: AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Astellas: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Lectures. Avivi: Novartis: Speakers Bureau; Kite, a Gilead Company: Speakers Bureau. Ram: Novartis: Honoraria; Gilead: Honoraria.
Off label Cellcepet for prophylaxis of GVHD