Abstract
Background:
Early Cytokine Release Syndrome (CRS) is a common complication following haploidentical stem cell transplantation (Haplo-HSCT) induced by the proliferation of alloreactive T-Cells. CRS is occurring more frequently in patients receiving peripheral blood stem cells (PBSC) comparatively to bone marrow transplant, however its impact on outcome, notably graft versus host disease (GVHD) remain unclear. The main objective was to evaluate the impact of severity of CRS on the risk of GVHD.
Patients and Methods:
This retrospective single-center study included patients who had received a first haplo-HSCT for hematological malignancies, with PBSC as graft source. All patients received either a reduced-intensity conditioning (RIC) based on thiotepa (5mg/kg), busulfan (260 mg/m²) and fludarabine (120 mg/m²) [TBF], or a non-myeloablative conditioning (NMAC) based on fludarabine (150 mg/m²), cyclophosphamide (29 mg/kg) and 2 Gy TBI [CyFluTBI]. GVHD prophylaxis was based on PT-Cy 50 mg/kg (day+3 and +4) and cyclosporine A plus mycophenolate mofetil starting at day+5. All patients were given GSCF from day+5 to neutrophil recovery.
Results:
241 consecutive patients were analyzed. One hundred patients (54%) had myeloid malignancies, and 111 (46%) had lymphoid malignancies. Most patients had intermediate or low risk DRI (n = 180, 75%) and HCT-CI was ≥ 3 for 159 patients (66%). Using ASTCT consensus criteria, 226 patients (94%) developed CRS, including 183 grade 1 and 43 grade ≥ 2. Transplantation and patient characteristics were not significantly different between patients with CRS grade 0-1 vs. ≥ 2, except for age. Indeed, patients with CRS grade ≥ 2 were significantly older than patients with CRS grade 0-1 (median 65 vs 60 yo respectively, p = 0.01).
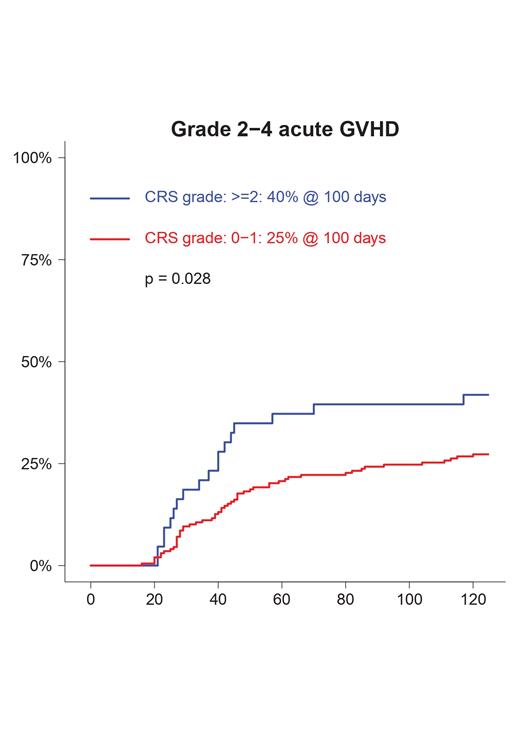
Patients with grade ≥ 2CRS had significantly higher cumulative incidence of day-100 grade II-IV acute GVHD (grade 0-1 vs. ≥ 2 : 28% and 44%, p = 0.028) and 4-year moderate to severe chronic GVHD (grade 0-1 vs. ≥ 2 : 16% and 30%, p = 0.024) compared to patients with grade 0-1 CRS (Figure 1). No difference in the cumulative incidence of relapse was observed between CRS groups (grade 0-1 vs. ≥ 2 : 22% and 21%, p = 0.802).
By multivariate analysis, CRS grade ≥ 2 was the only factor associated with grade II-IV acute GVHD (HR = 1.99; 95%CI = [1.17-3.39], p = 0.011). CRS grade ≥ 2 was significantly associated with a higher risk of moderate to severe chronic GVHD (HR = 2.67; 95%CI = [1.36-5.21], p = 0.004) and poorer GVHD- and relapse-free survival (GRFS) (HR = 1.78 ; 95%CI = [1.19-2.67], p = 0.005). Progression free survival, overall survival and non-relapse mortality were not influenced by the severity of CRS.
Conclusion:
In the context of PBSC haplo-HSCT, the occurrence of grade ≥ 2 CRS following graft infusion is significantly associated with an increased risk of both acute and chronic GVHD. This may improve the early identification of patients with high risk of GVHD for whom specific enhanced GVHD prophylaxis should be investigated.
Chabannon: Sanofi SA: Other: Travel Support, Research Funding, Speakers Bureau; Bellicum Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Speakers Bureau; Novartis: Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Terumo BCT: Speakers Bureau; Miltenyi Biotech: Research Funding; Fresenius Kabi: Research Funding; EBMT: Membership on an entity's Board of Directors or advisory committees. Blaise: Jazz Pharmaceuticals: Honoraria.