Abstract
Rational: The significant increase in haplo-identical allogeneic cell transplantation (Allo-HCT) has led to the more frequent use of cyclophosphamide (Cy) after transplantation for graft-versus-host disease (GVHD) prophylaxis (PT-Cy). This strategy has subsequently been used in related and unrelated allogeneic HCT settings as well with some controversial results.
Patients and Methods: We analyzed all consecutive Allo-HCTs from matched related (MR) and unrelated donors (HLA matched and mismatched: MUD and MMUD) reported to the SFGM-TC registry from January 2014 to December 2019 and who have received PT-Cy alone or in combination with other immunosuppressive molecules (IS). We therefore performed a pair-matched analysis (1/2) with transplantations using classic GVHD prophylaxis with (w) or without (wo) anti-thymocyte globulins (ATG). The primary objective was to evaluate the incidence and severity of acute and chronic GVHD in PT-Cy compared to other strategies. Secondary objectives included: modalities of PT-Cy and IS, cumulative incidence of relapse (CIR), non-relapse mortality (NRM), overall survival (OS), GVHD and relapse free-survival (GRFS), infection rates.
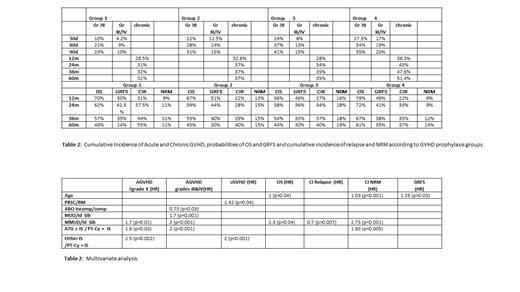
Results: We analyzed a total of 1190 patients (pts), 386 (32%) received PT-Cy and matched with 804 (68%) pts who received classic GVHD prophylaxis (no PT-Cy). Among PT-Cy patients, 59% were males with a median age of 55.3 years (3.4-75.5), 49% were AML, 14% ALL, 20% MDS and MPS, 12.5% NHL and HL, and 4.5% Multiple Myeloma. Before transplantation, 61% of pts were in complete remission (CR), 34% not in CR, 2% in stable disease and 3% were not treated. Conditioning regimen was myeloablative in 35% of patients, 86% received peripheral blood stem cells, 31% were CMV negative pairs, 58% were sex-matched and 51% were ABO compatible. There was no significant difference between the PT-Cy and no PT-Cy groups regarding all the variables. We identified four groups: group 1: PT-Cy + IS (n=259), group 2: PT-Cy + ATG + IS (n=120), group 3: ATG w or wo IS (n=651) and group 4: other IS (n=160). We observed significant differences between the 4 groups for age (p<0.001), type of disease (p<0.001), disease status (p=0.016), conditioning intensity (p=0.002), HC source (p<0.001), HLA matching (p<0.001) and ABO compatibility (p<0.001).
The cumulative incidence of acute GVHD grades II, III/IV, chronic GVHD, relapse and NRM, the probability of OS and GRFS are shown in Table 1. The results of multivariate analysis (Table 2) showed a significant lower incidence of acute GVHD gr II, III, IV and chronic GVHD after PT-Cy + IS and a significant higher TRM after ATG ± IS. In addition, other well-known parameters were found to have a significant impact as age on OS and GRFS, use of unrelated donor on OS and CIR and PBSC on chronic GVHD. Regarding severe infections after HCT, there was no difference between PT-cy and pair-matched patients except for pneumonia (12.5% vs. 8% respectively, p=0.08) and septicemia (12% vs. 1.7% respectively, p<0.001).
In conclusion, there was a significant lower incidence and severity of acute and chronic GVHD in the PT-Cy group after related, HLA matched and mismatched unrelated Allo-HCT compared to matched patients with other GVHD prophylaxis with a higher NRM when patients received ATG. We did not observe significant translation into GRFS improvement probably due to the higher toxicity of PT-Cy.
Yakoub-Agha: Jazz Pharmaceuticals: Honoraria. Forcade: Novartis: Other: travel grant. Chalandon: Incyte, BMS, Pfizer, Abbie, MSD, Roche, Novartis, Gilead, Amgen, Jazz, Astra Zenec: Other: Travel EXpenses, Accomodation; Incyte: Speakers Bureau; Incyte, BMS, Pfizer, Abbie, MSD, Roche, Novartis, Amgen: Other: Advisory Board. Huynh: Jazz Pharmaceuticals: Honoraria. Dulery: Novartis: Honoraria; Takeda: Consultancy; Gilead: Other: Travel support and registration fees for scientific meetings .