Abstract
INTRODUCTION :
HSCT remains the best curative treatment in eligible AML patients with adverse or intermediate cytogenetics according to the ELN classification, and for those with unfavorable molecular markers at diagnostic or with a positive measurable residual disease (MRD) after intensive chemotherapy. MRD-positivity after HSCT confers an inferior outcome and a recently published study demonstrated that the evaluation of WT1 MRD before HSCT or 3 months after HSCT is highly predictive of post-transplant relapse. Besides, the impact and the kinetics of MRD positivity on prognosis are still unclear. Here we assess the impact of both pre- and post-transplant MRD on long term survival and incidence of relapse among a real-life cohort of 117 AML patients who underwent HSCT.
PATIENTS AND METHOD:
From November 7, 2012 to December 17, 2019, 117 patients diagnosed with AML and aged over 18 years underwent HSCT in Amiens University Hospital Center. MRD status was assessed using RQ-PCR (sensitivity 10 -4 to 10 -5) or multiparametric flow cytometry (sensitivity 10 -3 to 10 -4) before HSCT, depending on the markers available at diagnosis, at day 30, day 100 and a year after HSCT. Overall survival (OS) and cumulative incidence of relapse (CIR) were evaluated at the same time-points in landmark analysis for patients surviving beyond d100 to reduce the impact of early death on CIR. Statistical analyses were performed with SPSS Statistics 20 (IBM).
RESULTS:
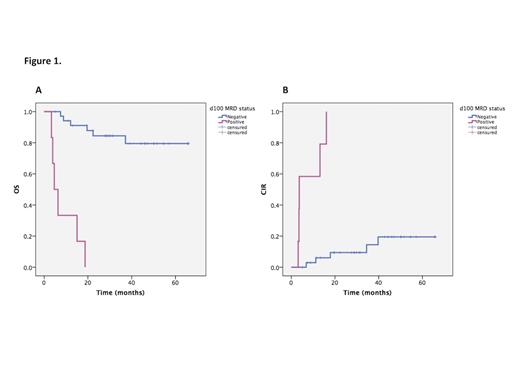
Median age was 55 years (range 18-72) with 67/117 males. 85% patients had de novo AML. Cytogenetic ELN risk stratification was favorable for 15 (13%), intermediate for 72 (62%) and adverse for 28 (25%). At the time of HSCT, 76% were in complete remission (CR) after intensive chemotherapy. 30 patients (25%) had myeloablative conditioning (MAC) or toxicities-reduced regimens (TRC), 34 patients (30%) had reduced-intensity regimens (RIC) and 53 patients (45%) underwent sequential-based conditioning regimens (Seq). For the 100 patients who survived beyond d100, median time follow-up was 23.5 months (3.0-85.0). OS at 1 and 3 years was respectively 80% (75.9-84.1) and 61.7% (56.1-67.3) and CIR at same time-points was 19,3% (15.2-23.4) and 29,8% (24.6-35.0). No OS and CIR differences were observed according to the conditioning regimen, stem cell source and ELN score. A progressive disease before HSCT increased the risk of relapse but didn't worsen the OS. Before HSCT, MRD was positive in 48 patients (58.5%) with analyzable markers, either at molecular or flow cytometry level. OS and CIR were not impacted by the pre-HSCT MRD. Strikingly, performing HSCT resulted in MRD negativation at day 100 for 85.4% of patients with previous positive MRD, significantly improving OS and CIR (p<.001 for both, Figure 1A and B). Beyond day 100, achieving a negative MRD limited the 5-year CIR to 20%. In multivariate analysis, OS was significantly inferior, in patients with acute GVHD > grade 2 (HR=5.4, 95CI=1.8-16.1; p=.002) and positive MRD at day 100 (HR=19.2, 95CI=6.0-61.1; p<.001). Positive MRD at day 100 was the only significant predictor of adverse CIR (HR=17.5, 95CI=5.6-55.3; p<.001).
DISCUSSION/CONCLUSION
In this real-life cohort of patients diagnosed with AML, we show that HSCT procedure consistently allows MRD negativation, subsequently improving the outcome of patients surviving beyond d100. Persistence of positive MRD after HSCT was the main predictor of long-term adverse outcome after transplant. Our data highlight the importance of MRD negativation at day 100 regardless of pre-transplant status, conditioning regimen or initial ELN classification.
No relevant conflicts of interest to declare.