Abstract
Treatment of pediatric acute lymphoblastic leukemia (ALL) has seen dramatic improvements over the last several decades, leading to survival rates of over 90% for specific groups. However, for patients with relapsed/refractory disease, achieving complete remission remains a significant challenge. To further improve treatment outcomes for these patients, improved functional screens are needed to better match high risk leukemia patients with novel therapeutic strategies.
Current functional screens are limited by the large patient samples required, particularly for combination drug screening and evaluation of higher order drug interactions. To that end, this work aims to develop a microfluidic multi-drug and multi-dose chemosensitivity assay to directly test candidate combinations of therapeutics in patient derived ALL cells. This will allow for analysis of response to combinations of 3 small molecule drugs while minimizing required patient sample and reducing experimental workload. Once validated, this assay can be used to identify biomarkers of response to novel combination regimens in pediatric ALL, and ultimately, could be used to prospectively guide therapy in newly diagnosed patients.
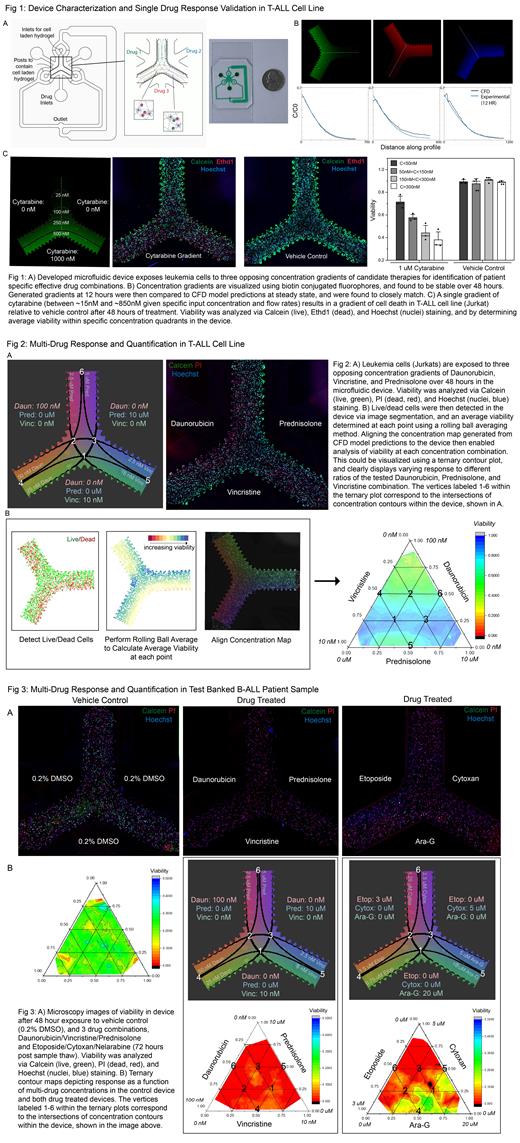
Specifically, this is accomplished by generating stable, overlapping concentration gradients of small molecule drugs across 3D cultures of leukemia cells with and without human bone marrow derived mesenchymal stem cells (Fig 1A). We have demonstrated the ability to generate and maintain these gradients over 48 hours, and have shown that experimentally generated concentration gradients closely match steady state computational fluid dynamics (CFD) model predictions (Fig 1B). Moreover, exposing a T-ALL cell line (Jurkats) to a single gradient of cytarabine results in a gradient in cell viability. In drug treated devices, increasing drug dose led to decreasing averaged viability, while no appreciable difference was seen in vehicle control devices (Fig 1C).
A commonly used 3 drug combination, Daunorubicin, Vincristine, and Prednisolone, was then used to validate the utility of the device for evaluating multi-dose drug combinations. Here, Jurkats were exposed to superimposed concentration gradients of Daunorubicin, Vincristine, and Prednisolone for 48 hours, after which cell viability as a function of concentration was examined. Specifically, a ternary contour map of the average viability across the device was generated, clearly depicting the range of response across the device (Fig 2B).
Previous results have demonstrated Jurkats' sensitivity to Daunorubicin as well as Vincristine and relative resistance to Prednisolone, and these results are closely recapitulated in the device. Previous reports in literature have also demonstrated potential for antagonism between anthracyclines and vinca alkaloids, specifically between Doxorubicin and Vincristine in Jurkats (Ehrhardt, 2011). Our results also suggest that some amount of antagonism may exist between Daunorubicin and Vincristine in Jurkats at these concentration ranges tested, evidenced by the high average viability in regions of high vincristine concentrations and mid-range daunorubicin concentrations.
Finally, we then used this system to evaluate response to drug combinations in a test patient sample. Specifically, combinations of Vincristine, Prednisolone, and Daunorubicin, as well as combinations of Nelarabine, Etoposide, and Cytoxan were evaluated in a standard risk B-ALL bone marrow aspirate sample. Drug treated devices resulted in significantly reduced viability relative to the vehicle control, with a differential in response observed between the 2 combinations tested (Fig 3A). Moreover, at these drug concentration ranges, it appears that combinations of Etoposide and Ara-G are particularly ineffective relative to other combinations in this sample (Fig 3B). Future studies will work to improve the baseline viability of banked samples in the device, and include testing of fresh leukemia samples as well.
We are also working to develop methods to use data collected from these devices to identify synergistic or antagonistic drug combinations, and in the future, can correlate this with clinical outcome metrics as well as biomarkers of response.
Tissue samples were provided by the Children's Healthcare of Atlanta Pediatric Bio-Repository. Other investigators may have received specimens from the same subjects.
Lam: Sanguina, Inc.: Current holder of individual stocks in a privately-held company.