Abstract
Introduction
The CHaDOx1 nCov-19 AstraZeneca (AZ) vaccination has been associated with an antibody-mediated prothrombotic syndrome, termed "Thrombosis with Thrombocytopenia Syndrome" (TTS)[1-3]. The current diagnostic criteria for TTS are thrombosis (venous or arterial) within 4-42 days of AZ vaccine, thrombocytopenia and presence of an antibody to platelet factor 4 (PF4)[4, 5]. TTS commonly presents with cerebral venous sinus thrombosis (CVST) or splanchnic vessel thrombosis (SVT), but outside of TTS, CVST and SVT are uncommon, with an overall incidence of less than 0.5 per 100,000 [5-7]. Deep vein thrombosis (DVT) and pulmonary embolism (PE) are also associated with TTS, however the background incidence of venous thromboembolism (VTE) is much higher, with 1-2 events per 1000 patients per year[7, 8]. Therefore, many patients will present with new VTE and a recent exposure to the AZ vaccine, requiring consideration of investigation for TTS. Recent data suggests that PF4 antibodies can be seen in up to 8% of patients without thrombosis but following AZ vaccination[9]. We hypothesised in patients with recent AZ vaccination, new VTE but with a normal platelet count, that the incidence of a PF4 antibody is similar to this background rate of PF4 positivity. If confirmed, then presence of a normal platelet count despite new VTE and recent vaccination may exclude TTS without the need for PF4 antibody testing. We present our preliminary data on the rates of PF4 antibody positivity amongst patients with VTE, recent AZ vaccination and a normal platelet count at presentation.
Aim and Methods
To assess the incidence of PF4 ELISA positive results in patients with confirmed VTE, recent vaccination (within 4-42 days) with the first dose of AZ vaccine, and platelet count greater than 150x10 9/L.
A retrospective audit of cases referred with suspected TTS to Monash Pathology, Melbourne, Victoria, and New South Wales Health Pathology at Royal Prince Alfred Hospital and St George Hospital sites Sydney, New South Wales, Australia, for testing for anti PF4 antibodies from 1 st April to 31 st July 2021.
Patient sera were tested for the Anti-PF4 antibody using the STAGO Asserachrom HPIA IgG ELISA (Asnières sur Seine, France). For patients with a positive PF4 antibody test additional testing was sought for either the presence of platelet activating antibodies with a flow cytometry-based assay or the presence of spontaneous serotonin release without heparin in the serotonin release assay.
Results
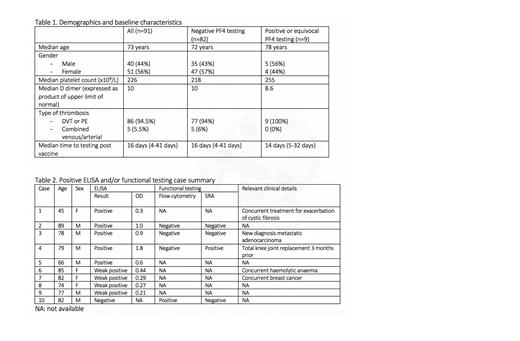
From April 1 st to July 31 st 350 tests were run on 332 patients. 91 patients met our criteria, of whom 51 were female and 40 male, with a median age of 73 years. Median platelet count at presentation was 226x10 9/L, and median D dimer values were 10 times the upper limit of normal. 86 patients had either DVT, PE or both, including 2 with upper limb DVT, and 5 patients had PE with concurrent arterial events (1 axillary artery thrombosis, 3 arterial strokes, 1 coronary artery thrombosis). Further details are presented in table 1. 82 patient samples tested negative for anti-PF4 antibodies by ELISA, 5 were positive, and were 4 weak positive/equivocal (see table 2 for further details). Of the positive results, 3 had functional testing available, of which 2 were negative, and 1 showed discordant results, with a positive SRA but negative flow cytometry. None of the weak positive/equivocal cases had functional testing results available. Of the negative ELISA results, 5 patients had functional testing results available, of which 4 were negative. One of these cases had positive testing by flow cytometry, but negative by SRA (case included in table 2).
Conclusion
In our Australian cohort of patients with their first dose of AZ vaccine and new VTE within 4-42days, but a normal platelet count (therefore not fulfilling the clinical criteria of TTS), the incidence of a positive PF4 antibody test was 9/91 (9.9%, 95% CI 3.7-15.9%) and only one had evidence of platelet activating antibodies. This observed rate is similar to that observed in healthy patients without thrombosis who received AZ vaccination as described by Thiele et. al., 2021. Further confirmation in a larger cohort of VTE patients is required, but if confirmed, then PF4 ELISA testing in patients with VTE and normal platelet count post AZ vaccine may not be required, and should give clinicians confidence to institute routine management.
No relevant conflicts of interest to declare.