Abstract
Background
Childhood acute lymphoblastic leukemia (ALL) has a very high cure rate, however, long-term survivors are at increased risk of chronic medical illnesses that are likely in part microbiome mediated. Previous studies comparing microbiota of pediatric ALL survivors to healthy siblings showed altered composition in survivors. Longitudinal microbiome changes through treatment leading to these alterations are unknown.
Methods
Children with ALL were enrolled and stool samples were collected at diagnosis and at the end of induction, consolidation, interim maintenance I, delayed intensification, interim maintenance II, as well as approximately 3 months and 6 months into maintenance. Stool samples from healthy siblings were used as controls. Clinical data were collected. DNA was extracted from stool samples and 16S rRNA was sequenced for analysis of hypervariable region V4. Differences in alpha and beta diversities and relative abundance of taxa were calculated between phases and with sibling controls.
Results
35 ALL patients age 3 months-19 years were included. The diagnoses were 14 standard risk pre-B ALL, 14 high risk pre-B ALL, 6 T-ALL, and 1 relapsed pre-B ALL. Stool samples were sequenced from 19 healthy siblings.
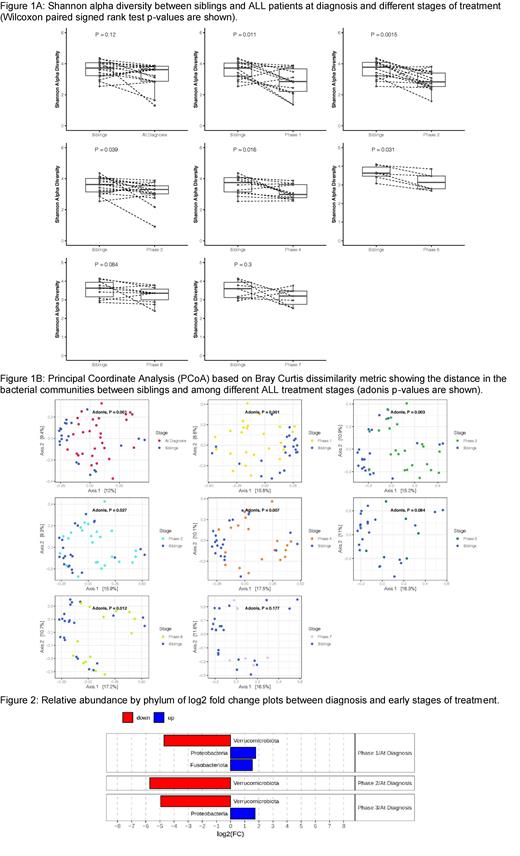
Statistically significant differences in alpha diversity (Shannon) were found between healthy siblings and ALL patients during the more intense chemotherapy phases before low dose maintenance (Figure 1A). Beta diversity (Bray-Curtis), was significantly different between microbiota of ALL patients at diagnosis and their siblings, as well as between ALL patients at diagnosis and at each of the subsequent treatment phases (Figure 1B). Longitudinal comparison using multivariate analysis showed that leukemia risk group (high risk vs standard risk) and antibiotic treatment were significant factors in beta diversity changes. The relative abundance of microbes showed that with treatment, ALL patients exhibit a significant decrease in the phylum Verrucomicrobiota, driven by the genus Akkermansia, which is beneficial to health and is associated with protection against obesity and other chronic inflammatory diseases (Figure 2). Proteobacteria and Fusobacteria, which include proinflammatory species, were increased.
Conclusion
Pediatric ALL patients have decreased diversity of gut microbes at diagnosis and during treatment. The types of gut microbes harbored in ALL patients are already different than their siblings at diagnosis and continue to change during treatment, but may begin to recover in low dose maintenance therapy. Taxa considered to be beneficial are depleted, while more pathogenic microbes become prominent. Microbiota changes are likely influenced by intensity of chemotherapy and antibiotic exposure. Continued longitudinal follow up is needed to determine whether these changes correlate with adverse long term health outcomes.
No relevant conflicts of interest to declare.