Abstract
Background
Venetoclax based therapies have produced varying results in the relapsed or refractory acute myeloid leukemia (R/R AML) setting. Highest response rates were demonstrated after Venetoclax in combination with high dose chemotherapy. However, this approach is feasible mainly in fit, younger patients. Herein, we present a lower intensity combination therapy consisting of Venetoclax, low dose Cytarabine and Actinomycin D (ACTIVE) in patients with R/R AML administered in real-life clinical practice setting.
Methods
We performed an observational, retrospective, single centre study. The patients were at least 18 years of age and had R/R AML. All patients provided informed consent for treatment as well as data collection. Venetoclax ramp-up was administered over either 3 or 5 days until the daily dose of 600mg/d was reached. The ACTIVE regimen consisted of Venetoclax 600mg/d p/o on days 1-28, Cytarabine 20mg/m 2 s/c on days 1-10, Actinomycin D 12.5 µg/kg i/v on days 1, 2 and 3 (on days 1 and 2 for patients ≥65 years). Strong/moderate CYP3A inhibitors/inducers or Venetoclax dose reductions were not allowed. Indications for stopping Venetoclax before Day 28 were life threatening infectious complications or faster hematological recovery with the addition of G-CSF in responders. A second ACTIVE cycle was administered in non-responders without evidence of progressive disease after Cycle 1 or in responders with positive minimal residual disease (MRD).
We evaluated baseline characteristics, composite CR (CRc = CR + CRi + CRp), overall response (ORR = CRc + MLFS), MRD negativity rates, overall survival (OS), relapse-free survival (RFS), event-free survival (EFS), Grade 3-5 non-hematological toxicities and Day 30 and Day 60 mortality rates.
Results
Fifty R/R AML patients were treated with ACTIVE and 56% (28/50) were male. The median age was 65 years (20-84). The median Eastern Cooperative Oncology Group (ECOG) status was 1 (0-3) and 40% (20/50) had ECOG status of 2 or 3. Secondary AML was confirmed in 48% (24/50) of cases. Adverse cytogenetics were identified in 28% (14/50) of patients whereas 60% (30/50) were stratified to adverse risk group in accordance with ELN 2017 guidelines. The most common gene mutations were IDH1/2 30% (15/50), FLT3 28% (14/50), ASXL1 22% (11/50), NPM1 14% (7/50), N/KRAS 12% (6/50) and RUNX1 12% (6/50).
The median number of prior therapies was 2 (1-5). Intensive chemotherapy was administered in 80% (40/50) of whom 38% (15/40) had primary refractory disease and 48% (19/40) had previously received Fludarabine + Cytarabine + Idarubicin (FLAG-Ida) or Cytarabine + Mitoxantrone (HAM). Eight percent (4/50) had had prior Venetoclax exposure. Thirty-six percent (18/50) had relapsed after allogeneic stem cell transplantation (alloSCT) with a median time of 7.4 months (1.6-37.3) from transplant to relapse.
One cycle of ACTIVE therapy was administered in 76% (38/50) of cases, whereas 24% (12/50) received 2 cycles. The median number of Venetoclax 600mg/d days per cycle was 18 (8-28). Additional FLT3/RAS/BCR-ABL1 inhibitors Gilteritinib, Trametinib or Dasatinib were administered in 12% (6/50).
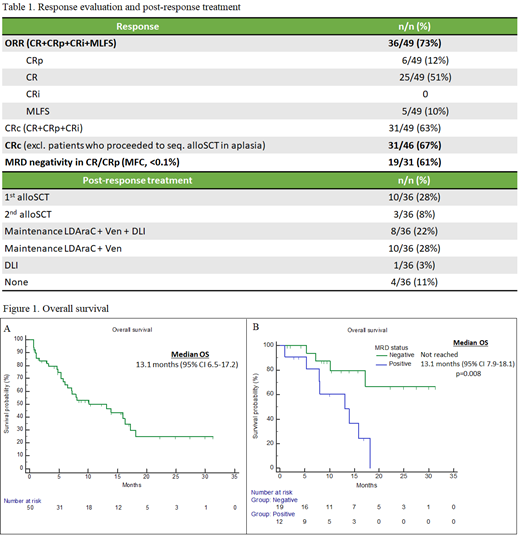
Forty-nine patients were evaluable for response and one patient died of sepsis before response evaluation (Table 1). The ORR was 73% (36/49) and the CRc rate was 67% (31/46). MRD negativity was confirmed in 61% (19/31) of CRc patients. Sixteen patients had undergone additional early bone marrow evaluations. Blast count reduction to <5% was observed in 50% (8/16) after Venetoclax ramp-up and in 88% (14/16) on Day 4.
Half of the ACTIVE responders (18/36) continued maintenance therapy with Venetoclax + low dose Cytarabine and optional DLI, whereas 36% (13/36) proceeded to either first or second alloSCT. After 16.4 months of median follow-up, the median OS, RFS and EFS were 13.1, 7.2 and 4.5 months, respectively (Figure 1A). Median OS was not reached in MRD negative patients (Figure 1B). In multivariable Cox regression analysis, adverse cytogenetics (HR 3.48, 95% CI 1.39 -8.55) and primary refractory disease (HR 2.54, 95% CI 1.05-6.12) were associated with worse OS.
The most common grade 3-5 non-hematological adverse events were febrile neutropenia (54%, 27/50) and bacteremia/sepsis (34%, 17/50). Day 30 and Day 60 mortality rates were 8% (4/50) and 16% (8/50), respectively.
Conclusions
ACTIVE was effective and well tolerated in this unselected prognostically unfavourable older R/R AML patient population.
Zucenka: Jannsen: Honoraria, Other: Travel-expenses; Takeda: Other: Travel Expenses; Novartis: Honoraria, Other: Travel Expenses; Pfizer: Honoraria, Other: Travel Expenses; Astellas: Honoraria; Abbvie: Honoraria, Other: Travel Expenses. Maneikis: Abbvie: Honoraria. Pileckytė: Abbvie: Honoraria, Other: Travel Expenses. Griškevičius: Abbvie: Other: Travel Expenses.
Venetoclax has been used off-label for the treatment of R/R AML