Abstract
Background Although European Leukemia Net (ELN) risk classification was introduced in 2017 and has been applied as an important prediction tool for prognosis, there has been limited data on its value among the patients with allogeneic hematopoietic stem cell transplantation (HSCT). We evaluated the prognostic value of ENL 2017 criteria on post-HSCT outcomes and compared it with pre-HSCT measurable residual disease (MRD) status determined by Wilms tumor gene 1 (WT1) expression level.
Methods: Patients who underwent HSCT and fulfilled following criteria were eligible in this current study: first HSCT in complete remission (CR) or CR with incomplete hematologic recovery and having bone marrow WT1 expression results before transplant. We found a total of 275 patients between Nov 2017 and July 2020, and we adopted the WT1 cut-off level of 250 copies per 10 4 ABL for defining MRD negative vs positive (Biol Blood Marrow Transplant. 2019;25:1925) .
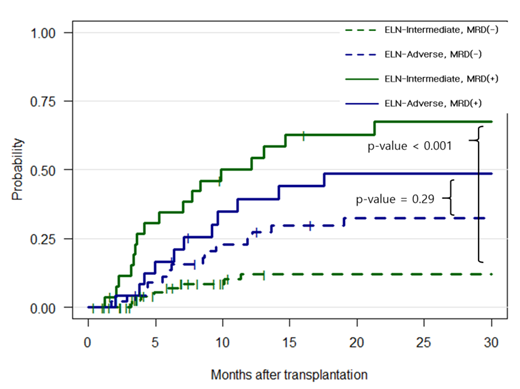
Results: Among 180 patients, , 110 (61%) and 70 (39%) patients were classified as a , intermediated (INT) and adverse (ADV) risk group by ELN 2017 classification. After a median follow-up of 18.3 months (range, 0.4 to 43.2 months), the Kaplan-Meier survival curve could not discriminate overall survival (OS), relapse free survival (RFS), or cumulative incidence of relapse (CIR) between the INT and ADV risk groups (p=0.2, p=0.68, p=0.061, respectively). On the other hand, we found that OS, RFS and CIR were unfavorable in MRD (+) group compared to either MRD negative INT or ADV risk group (35.8 % vs 59.1 % for OS, p=0.05; 24.7% vs 55.9% for RFS, p=0.002; 60.9% vs 20.4 % for CIR, p<0.001). We further divided the groups into 4 subgroups with incorporating pre-HSCT WT1 level: INT MRD(-), INT MRD(+), ADV MRD(-), and ADV MRD(+). Notably, the importance of MRD was more prominent in the INT risk group with showing significant differences in CIR between INT MRD(-) and INT MRD(+) group (p<0.001) in contrast to that observed between ADV MRD(-) and ADV MRD(+) groups (p=0.12). Among the 4 subgroups, patients of INT MRD(+) confers worst prognosis in regards to OS, RFS and CIR, which was even worse than those of ADV MRD(+) group.
Conclusions: The ELN 2017 risk classification was not available to predict post-HSCT outcomes in INT and ADV risk group. We found that pre-HSCT MRD rather than ELN 2017 could more likely to predict post-HSCT relapse. The prognostic value of WT1 MRD was more prominent in ELN INT group compared to ADV group. A subset of INT patients had the worst prognosis if their pre-HSCT WT1 MRD remained positive, who they need additional therapeutic strategies to prevent relapse.
Kim: Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; ILYANG: Research Funding; Takeda: Research Funding. Kim: AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AIMS Biosciense: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AML-Hub: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BL & H: Research Funding; BMS & Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boryung Pharm Co.: Consultancy; Daiichi Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Handok: Consultancy, Honoraria; LG Chem: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria; Pintherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi Genzyme: Honoraria, Speakers Bureau; SL VaxiGen: Consultancy, Honoraria; VigenCell: Consultancy, Honoraria.