Abstract
Introduction: Spleen and liver stiffness, investigated by transient elastography (TE), have been associated with marrow fibrosis in patients (pts) with Ph-negative myeloproliferative neoplasms (MPNs) (Iurlo et al, Br J Haematol. 2015; Webb et al, Ultrasound Q. 2015). Morover, spleen stiffness was found to be greater in Myelofibrosis (MF) and Polycythemia Vera (PV) compared to Essential Thrombocythemia (ET) (Benedetti et al, J Clin Med. 2020). Tissue stiffness can be assessed by ultrasound shear wave elastography (SWE), the two most common techniques being point SWE (pSWE) and bidimensional SWE (2D.SWE).
Aims: The aims of this study are: 1) to identify TE differences between MPN pts and healthy volunteers (HV); 2) to evaluate specific TE features in pts with MF, PV and ET; 3) to assess whether spleen/liver stiffness may identify clinical-laboratory features associated with prognosis in MPNs
Methods: In this monocentric study, MPN pts and HV received elastometric evaluation of spleen and liver stiffness by pSWE and 2D.SWE with an Esaote MyLab™9 ultrasound system. Spleen area, portal (PVD) and splenic vein diameter (SVD) were measured.
Results: A total of 220 pts were included in this study: 142 (64.5%) MPN and 78 (35.5%) HV. MPN pts were affected by MF (63, 44.4%: 39 primary MF), PV (33, 23.2%) or ET (46, 32.4%).
Compared to HV, MPN pts had greater median spleen maximal cross sectional area (79 vs 38 cm2, p<0.001), greater spleen stiffness (pSWE 31.3 vs 23.7 kPa, p<0.001; 2D.SWE 25.2 vs 18.7 kPa, p<0.001), and greater liver stiffness (pSWE 6.0 vs 4.9 kPa, p<0.001; 2D.SWE 5.4 vs 4.7 kPa, p<0.001). Additionally, PVD and SVD were significantly larger in MPNs than in HV (PVD 10.9 vs 9.2 mm, p<0.001; SVD 8 vs 6.3 mm, p<0.001). Comparing each MPN to HV, only MF retained all the significant differences; conversely, liver stiffness and PVD were comparable between ET/PV and HV.
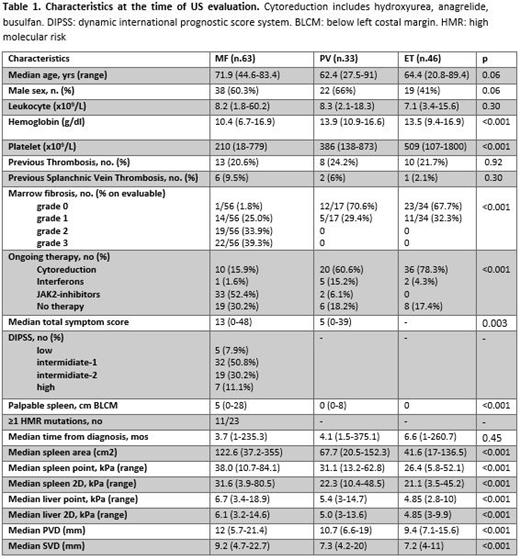
Clinical and laboratory features of MPN pts are shown in Tab 1. Compared to PV and ET pts, MF pts had higher spleen (p<0.001) and liver stiffness (p<0.001), larger PVD (p<0.001) and SVD (p<0.001). Conversely, ET and PV displayed comparable TE values.
Notably, higher median spleen area (p<0.001), larger SVD (p=0.03) and PVD (p=0.02), higher liver (pSWE/2D.SWE, p<0.001/p=0.002) and spleen stiffness (pSWE/2D.SWE, p=0.01/p=0.001) were associated with increased marrow fibrosis grade. Grade 0-1 marrow fibrosis was present in 15 MF, 17 PV and 34 ET pts. Considering only these 66 MPN pts, spleen (40.8 vs 31.3/25.6 in PV/ET, p=0.006) and liver (6.5 vs 5.6/4.7 in PV/ET, p=0.01) stiffness was significantly higher in MF pts.
Notably, increased spleen fibrosis was significantly associated with thrombotic history (32.2 vs 24.3 kPa in pts without previous thrombosis, p=0.02). Also, MPN pts with splanchnic vein thrombosis had higher spleen (pSWE: p<0.001; 2D.SWE: p<0.001) and liver stiffness (pSWE: p <0.001), and increased PVD (p=0.02) and spleen area (p=0003).
In MF pts, TE data did not correlate with DIPSS risk category. However, a higher spleen stiffness (pSWE/2D.SWE, p=0.09/ p=0.03), liver stiffness (pSWE/2D.SWE, p=0.001/p=0.01), PVD (p=0.002), and SVD (p=0.01) were associated with larger spleen length by palpation. Also, a reduced SVD was associated with the presence of ≥1 high molecular risk mutation (HMR) (p=0.04). As expected, MF pts treated with JAK-inhibitors showed larger spleen area (143.8 vs 83.7 cm 2, p=0.01) and higher spleen stiffness (34.3 vs 24 kPa, p=0.01) compared to pts under cytoreductive therapy. However, pts in spleen response at the time of TE had lower median SVD/PVD (p=0.05/p=0.07) and reduced spleen stiffness (sSWE/2D.SWE: 31.5/25.9 vs 39.0/32.8 in non-responders, p=0.01/p=0.04)
In ET/PV, TE data were comparable in pts with/without a complete hematological response. However, IFN was associated with enlarged spleen area and stiffness compared to cytoreduction.
Conclusions: TE evaluation effectively distinguishes MF pts from HV and ET/PV, while ET/PV show relevant similarities to each other and to HV. TE data were significantly associated with prognostically relevant features including marrow fibrosis and history of thrombosis in all MPNs, and presence of large splenomegaly and HMR in MF. Finally, TE data were significantly associated with spleen response in MF. Overall, spleen/liver stiffness may help in correct MPN diagnosis, and may provide clinical guidance, being associated with known prognostic factors and treatment outcome.
Cavo: Bristol-Myers Squib: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotechnologies: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Accommodations, Speakers Bureau. Piscaglia: ESAOTE: Research Funding. Palandri: CTI: Consultancy; AOP: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract