Abstract
Background: Bruton tyrosine kinase (BTK) inhibitors are a preferred treatment option in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Acalabrutinib (acala) is a next-generation, highly selective, covalent BTK inhibitor approved for the treatment of patients with CLL including those with R/R disease. In the primary analysis of the ASCEND study with a median follow-up duration of 16.1 months, acala monotherapy demonstrated superior progression-free survival (PFS) compared with idelalisib (Id) plus rituximab (R) (IdR) or bendamustine (B) plus R (BR) and favorable safety in patients with R/R CLL (Ghia et al. J Clin Oncol. 2020;38:2849-2861). Herein we report results of the ASCEND study at 3 years of follow-up.
Methods: In this randomized, multicenter, open-label, phase 3 study (NCT02970318), patients with R/R CLL were randomized 1:1 to receive acala 100 mg orally (PO) twice daily or investigator's (INV) choice of IdR (Id: 150 mg PO twice daily; R: 375 x1 then 500 mg/m 2 intravenously [IV] for 8 total infusions) or BR (B: 70 mg/m 2 IV and R: 375 x1 then 500 mg/m 2 IV for 6 total cycles) until disease progression or unacceptable toxicity. Crossover to the acala monotherapy arm was permitted in patients who progressed on IdR or BR. Assessments included INV-assessed PFS, overall survival (OS), INV-assessed overall response rate (ORR), and safety.
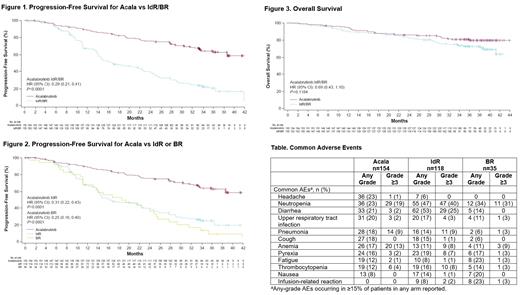
Results: A total of 310 patients (acala, n=155; IdR, n=119; BR, n=36) were enrolled (median age: 67 y; del(17p) 16%, unmutated IGHV 78%, Rai stage 3/4 42%). At a median (range) follow-up of 36.0 (0.5-44.0) and 35.2 (0.03-42.5) months (data cutoff: October 26, 2020) for acala and IdR/BR, respectively, acala significantly prolonged INV-assessed PFS vs IdR/BR (median: not reached [NR] vs 16.8 months, respectively; hazard ratio [HR]: 0.29; 95% confidence interval [CI]: 0.21, 0.41; P<0.0001); 36-month PFS rates were 63% for acala vs 21% for IdR/BR (Figure 1). Similar PFS benefits were observed for acala vs IdR (median: 16.2 months [HR: 0.31; P<0.0001]) and vs BR (median: 18.6 months [HR: 0.25; P<0.0001]) when assessed separately (Figure 2). PFS benefit was also consistently shown in high-risk subgroups; in patients with the del(17p) mutation, median PFS was NR vs 13.8 months (HR: 0.13; 95% CI: 0.06, 0.3; P<0.0001); 36-month PFS rates were 66% and 5% for acala and IdR/BR, respectively. In patients with unmutated IGHV, median PFS was NR vs 16.1 months (HR: 0.30; 95% CI: 0.21, 0.42; P<0.0001); 36-month PFS rates were 61% and 17% for acala and IdR/BR, respectively. Median OS was NR in both arms; the 36-month OS rate was 80% for acala vs 73% for IdR/BR (Figure 3). ORR was 83% with acala vs 85% with IdR/BR (ORR including partial response with lymphocytosis was 92% vs 88%, respectively).
Adverse events (AEs) occurring in ≥15% of patients in any treatment arm are shown in the Table; the most commonly reported all-grade AEs (≥20%) with acala were headache (23%), neutropenia (23%), diarrhea (21%), and upper respiratory tract infection (20%); with IdR, diarrhea (53%) and neutropenia (47%); and with BR, neutropenia (34%), fatigue (23%), infusion-related reaction (23%), and nausea (20%). Serious AEs (SAEs) were reported in 38% of acala, 63% of IdR, and 26% of BR patients; SAEs reported in ≥5% of patients in any treatment arm were pneumonia (acala 8%, IdR 9%, BR 3%), pyrexia (acala 2%, IdR 7%, BR 3%), and diarrhea (acala 1%, IdR 15%, BR 0%). AEs led to drug discontinuation in 21% of acala, 65% of IdR, and 17% of BR patients. Events of clinical interest included all-grade atrial fibrillation/flutter (acala 6%, IdR/BR 3%), all-grade hypertension (acala 7%, IdR/BR 4%), all-grade major hemorrhage (acala 3%, IdR/BR 3%), grade ≥3 infections (acala 25%, IdR/BR 27%), and all-grade second primary malignancies excluding non-melanoma skin cancer (acala 7%, IdR/BR 3%).
Conclusions: At 3 years of follow-up, the efficacy of acala monotherapy was maintained, showing a significant PFS benefit over standard-of-care regimens in patients with R/R CLL. Acala also maintained an acceptable tolerability profile with no new safety findings identified with longer-term follow-up.
Jurczak: AbbVie, AstraZeneca, Bayer, BeiGene, Celtrion, Celgene, Debbiopharm, Epizyme, Incyte, Janssen, Loxo Oncology, Merck, Mei Pharma, Morphosys, Novo Nordisk, Roche, Sandoz, Takeda, TG Therapeutics, Pharmacyclics, Affirmed, Gilead Sciences, Nordic Nanovecto: Research Funding; AstraZeneca, BeiGene, Janssen, Loxo Oncology, Sandoz, Roche: Membership on an entity's Board of Directors or advisory committees; European Medicines Agency, Sandoz-Novartis, Janssen China R&D, BeiGene, Epizyme, Acerta, AstraZeneca: Consultancy; Jagiellonian University: Ended employment in the past 24 months; Maria Sklodowska-Curie National Research Institute of Oncology: Current Employment. Pluta: Celgene, Servier, Takeda, Novartis: Honoraria; Celgene: Other: Travel, Accommodations, Expenses; Janssen-Cilag, Kartos Therapeutics, Iqvia, Roche, Acerta Pharma, Pharmacyclics, BeiGene, Takeda: Research Funding; National University of Sanok: Current Employment; Szpital Specjalistyczny w Brzozowie: Ended employment in the past 24 months. Lysak: Novartis, Janssen-Cilag, AbbVie; AstraZeneca: Honoraria, Research Funding. Šimkovič: AbbVie, AstraZeneca, Janssen-Cilag, Gilead, Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants. Kriachok: Takeda, Roche, Abbivie, Janssen, MSD: Consultancy; Takeda, Roche, Abbvie, Janssen, MSD, Pfizer: Honoraria, Speakers Bureau. Illes: Novartis, Janssen, Pfizer, Roche: Other: Travel, Accommodations, Expenses; Takeda, Seattle Genetics: Research Funding; Janssen, Celgene, Novartis, Pfizer, Takeda, Roche: Consultancy. de la Serna: ABBVIE, ASTRAZENECA,ROCHE: Research Funding; AbbVie, AstraZeneca, Beigene, Gilead, GSK, Janssen, Jazzpharma, Novartis, Roche: Consultancy; AbbVie, AstraZeneca, Roche: Speakers Bureau. Campbell: Novartis: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; CSL Behring: Consultancy; AstraZeneca: Consultancy; Amgen: Consultancy, Research Funding; BMS/Celgene: Research Funding; Roche: Consultancy, Research Funding. Musuraca: Janssen, Roche, Incyte: Honoraria; Janssen, Roche, Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen, Incyte, Roche: Consultancy. Jacob: Astrazeneca, GlaxoSmithKilne: Current equity holder in publicly-traded company; Horizon Discovery, Oxford Biomedica: Divested equity in a private or publicly-traded company in the past 24 months; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Clinical Haematology Services: Membership on an entity's Board of Directors or advisory committees; AbbVie, Astrazeneca: Honoraria. Avery: Macrogenics, Moderna: Divested equity in a private or publicly-traded company in the past 24 months. Wang: AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Yu: AstraZeneca: Current Employment; EMD Serono Research Institute: Ended employment in the past 24 months; AstraZeneca, Johnson and Johnson, AbbVie, Abbott: Current equity holder in publicly-traded company; Merck KGaA: Divested equity in a private or publicly-traded company in the past 24 months. Ghia: AbbVie: Consultancy, Honoraria, Research Funding; Acerta/AstraZeneca: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; ArQule/MSD: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Celgene/Juno/BMS: Consultancy, Honoraria; Gilead: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Sunesis: Research Funding.