Abstract
Introduction: As of July 31, 2021, 5 CAR-T therapies have been approved in the U.S. to treat lymphomas (large B cell, follicular, and mantle cell), acute lymphoblastic leukemia, and multiple myeloma. These individualized, autologous cell therapies are expensive; involve complex manufacturing, transportation, and storage requirements; and have a unique set of serious adverse events associated with their use. Thus, the use of CAR-T therapies is limited mainly to tertiary care centers that have been licensed to administer these agents. Over half of all U.S. patients with cancer are treated in community-based oncology practices. Understanding the adoption of novel therapies by community hematologists/oncologists (cH/O) is critical to assessing their future utilization and impact on clinical outcomes in the real-world setting. We have assessed CAR-T adoption among cH/O in lymphoma (Gajra et al. Immunotherapy. PMID: 32552151) and with the expansion in number of agents and indications, we sought to assess the evolution of their use of, referrals for, and barriers to CAR-T therapy.
Methods: Between January and April 2021, oncologists from across the U.S. were invited to complete a web-based survey about their CAR-T therapy utilization, referral patterns, barriers experienced, and overall impression of this class of therapy. Participant demographics and practice characteristics were also captured in the survey. Participants were not aware of the specific content of the survey as other areas in hematology/oncology were also addressed. Participants were compensated for their participation. Responses were aggregated and analyzed using descriptive statistics.
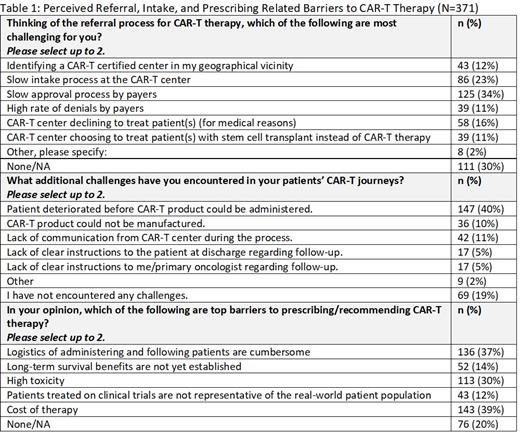
Results: Of the 371 cH/O invited, 100% completed the survey; 63% identified their primary specialty as hematology oncology and 36% medical oncology. The median time in practice was 16 years with a median of 20 patients seen per day on clinic days. The top 3 hematologic malignancies treated by survey participants are CLL, AML, and B-cell NHL. Among the participants, 72% and 53% have referred patients for CAR-T therapy since the first approval and the preceding 6 months respectively; 16% of those who referred in preceding 6 months, reported that none of their referrals ultimately received the CAR-T product infusion.
The top 2 barriers impacting timely referral for these participants include a slow approval process by payers (34%) and a slow intake process at the CAR-T center itself (23%). While patient deterioration is a significant challenge to 40% of the participants, other CAR-T center-related challenges perceived by the participants include the CAR-T product not being manufactured, lack of communication from the CAR-T center during the process, and lack of clear instructions to the referring oncologist about follow-up care. Common perceived barriers to prescribing/recommending CAR-T therapy include lack of long-term survival data (14%), therapy-related toxicity (30%), cumbersome logistics of administration (37%), and cost (39%). Among 309 participants queried about cost, 60% feel the price is acceptable for this breakthrough therapy.
To facilitate their prescribing/recommendation of CAR-T therapies, the participants agreed that better support to community providers regarding post therapy management, easier referral process to CAR-T centers, timelier approval from payers, and more long-term clinical trial and real-world data are needed. As more CAR-T therapies gain approval and come to market, additional resources requested by the participants included more financial aid/support for patients (59%), more education for prescribing physicians (53%) and patients (29%), more decision support tools (38%), and better coordination and communication between community physicians and sites of care (37%).
Conclusions: Most cH/O surveyed have referred patients for CAR-T therapies in hematologic malignancies but there is room for improvement in the referral and intake process as well as the treatment and post-infusion phase. Significant barriers of logistics and cost are potential deterrents to appropriate use. cH/O would welcome additional resources to aid the utilization of CAR-T. These results can inform stakeholders (manufacturers, payers, hospitals, and practices) regarding the need to improve processes and develop payment models to address cost in order to facilitate access of these agents to the appropriate patients.
Gajra: Cardinal Health: Current Employment, Current equity holder in publicly-traded company. Jeune-Smith: Cardinal Health: Current Employment. Feinberg: Cardinal Health: Current Employment.