Abstract
Introduction
The current International Society on Thrombosis and Hemostasis (ISTH) guideline of Thrombotic Thrombocytopenic Purpura (TTP) recommends Therapeutic Plasma exchange (TPE) and corticosteroid in the management of TTP, with Rituximab and Caplasizumab as additional potential therapies [X Long Zheng et al, J Thromb Haemost. 2020;18:2496-2502]. This potentially fatal condition requires prompt diagnosis and treatment but large volume plasma treatment is not without risk and in settings with limited access with sufficient compatible plasma for apheretic exchange, this can delay this emergency therapy. We have been running the first therapeutic apheresis services with an Optia instrument in Uganda at the Joint Clinical Research Centre in Kampala city and TTP is the main indication for TPE. We present 2 recent sequential cases of TTP that were successfully managed when Bortezomib was combined with plasma therapy.
Case1.
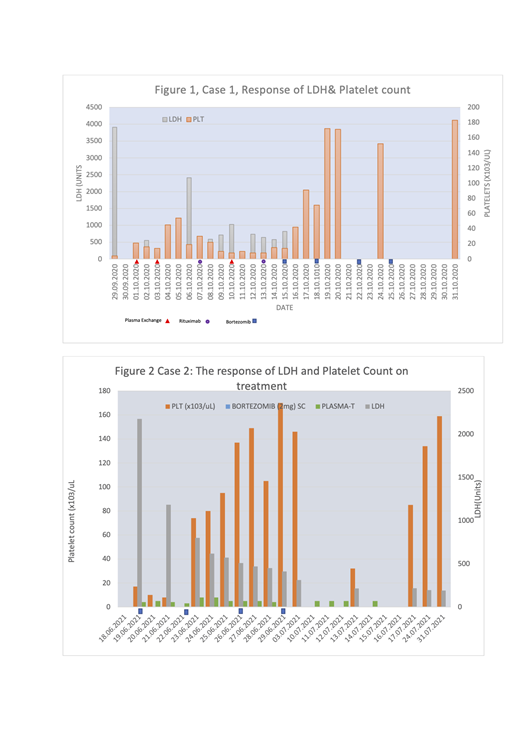
A 25yr woman referred to our service on 29/09/2020, with recent onset of bruising, icterus, severe anemia Hgb5.7g/dL, Severe thrombocytopenia 8 x 10 3/uL, a high LDH 3909U with schistocytes on the peripheral blood film severe thrombocytopenia and a negative Direct Antiglobulin test. She was Blood Group B+. She was managed with 3 sessions of TPE of 2.1, 1.1 and 2.0 plasma volume exchanges with 6727mL, 3026mL and 5459mL of replacement plasma respectively and oral prednisone 60mg daily without remission. Subsequent quantities of plasma were insufficient for apheresis and were instead transfused. She also received weekly Rituximab 500mg (IV) but without significant recovery in the platelet count until she received Bortezomib 2mg (SC) when there was corresponding recovery of the platelet count and with each dose to a total of 4 doses (Figure 1).
Case2.
A 55yr man was previously well and shopping in a mall when he had an index episode of seizures on 19.Jun.2021 and was hospitalized that day with altered consciousness and focal motor signs. He required intubation for 3 days and at the time of hospitalization, he had Hgb 9gm/dL, PLT 17 x 10 3/uL, LDH2177U, with numerous schistocytes on the peripheral blood film and a negative Direct Antiglobulin Test. The baseline blood sample functional ADAMTS13 was 3% and had Blood Group AB+. He had received the 1 st dose of the Astra-Zeneca Covid19 vaccine on 06.May.2021. It was not possible to get any AB plasma and we considered it risky to perform large volume TPE with non-AB plasma and he was instead treated with daily transfusion with Group A+ Plasma concurrent with daily Prednisone 60mg. Because of our previous experience of poor response to Rituximab, we opted to treat him upfront with 4 doses of 2mg bortezomib (SC) given 3 days apart. He made brisk recovery to complete remission and was discharged for weekly outpatient CBC and LDH monitoring. His platelet count subsequently dropped without a corresponding drop in the Hgb and no rise in the Hgb. We initially re-hospitalized him for 4 more days of plasma transfusion but without a brisk response and since the LDH was not increasing, the isolated Thrombocytopenia was considered a side effect of Bortezomib that was expected to resolve and he receive no further treatment but continued to recover to complete remission as an out-patient (figure 2).
Discussion:
Our findings suggest a possible role for Bortezomib in frontline therapy for TTP with potential to reduce the plasma requirement in TPE and this approach warrants a randomized clinical trial.
No relevant conflicts of interest to declare.
Bortezomib Indicated in the management of Multiple Myeloma and Plasma Cell Dyscrasia