Abstract
Background: The optimal induction strategy for patients with acute myeloid leukemia (AML) older than 65 years remains unknown. The use of lower intensity therapies in this population has become routine. The inclusion of venetoclax to intensify a hypomethylating agent backbone has efficacy outcomes approaching those of anthracycline-based inductions in elderly adults. This retrospective analysis sought to compare venetoclax-based induction combinations to anthracycline-based regimens.
Patients & Methods: We retrospectively analyzed all patients with AML older than 65 treated with 7+3 or liposomal daunorubicin and cytarabine (CPX-351) versus venetoclax with either decitabine or azacitidine from June 2018 to December 2020 at our institution. Patients were included in the analysis only if ECOG performance status prior to induction supported intensive induction (≤2). Baseline patient demographics, cytogenetic risk, molecular profiling (through next-generation sequencing), toxicity, responses, and MRD analysis were obtained. Kaplan-Meier curves were constructed using censoring at date of last contact for those that did not have a death event in the specified time period.
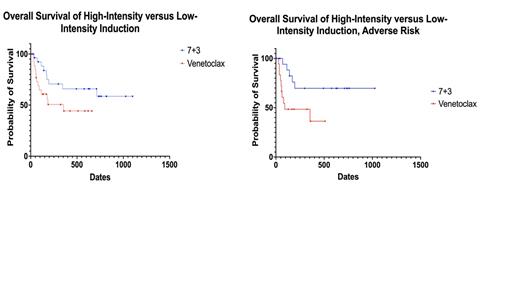
Results: The intensive cohort included 28 patients, 15 (53.6%) males and 13 (46.4%) females. The median age at diagnosis was 69 (range: 65 - 76), the median ECOG score was 1, and the median CCI score was 6. The anthracycline in the 7+3 cohort was daunorubicin in 15 patients (53.6%) and idarubicin in 1 (3.6%) patient. This cohort also included 12 (42.9%) patients treated with CPX-351. Two (7.1%) patients had favorable cytogenetics, 6 (21.4%) had intermediate cytogenetics, and 20 (71.4%) had adverse cytogenetics. The most common non-hematological toxicities that Grade 1 or higher were neutropenic fever (85.7%), infection (71.4%), GI/hepatobiliary (42.9%), cardiovascular (35.7%), and acute kidney injury (32.1%). One (3.8%) of the 26 evaluable patients died within 30 days and one (3.8%) died within 60 days. Fourteen patients (50.0%) achieved CR and 3 (10.7%) achieved CRi for an ORR of 60.7%. Six of the 17 patients with CR or CRi were analyzed for MRD and two (33.3%) were MRD-negative. Six (21.4%) received an allogenic stem cell transplant (SCT). The median OS in the intensive cohort was not reached at a median follow-up of 711 days (23.4 months).
The lower intensity cohort totaled 28 patients, 18 (64.3%) males and 10 (35.7%) females. The median age at diagnosis was 75 (range: 65 - 85), median ECOG score was 1, and the median CCI score was 6. There were no differences in ECOG score (p = 0.930), CCI (p = 0.195), or cytogenetic risk (p = 0.924) between the two groups. Venetoclax was given in combination with 5-day decitabine (78.6%) or azacitidine (21.4%). Of the 28 patients with evaluable cytogenetics, 2 (7.1%) were favorable, 5 (17.9%) were intermediate, and 19 (67.9%) were adverse. The most common non-hematological toxicities were infection (57.1%), neutropenic fever (50.0%), GI/hepatobiliary (28.5%), cardiovascular (25.0%), and acute kidney injury (21.4%). Of the 28 evaluable patients, one (3.6%) died within 30 days and 7 (25.0%) died within 60 days, though not statistically significant compared to the intensive group (p = 0.103). Two (7.1%) achieved CR and 12 (39.3%) achieved CRi for an ORR of 46.4%. Two of the 13 patients were evaluated for MRD and none were MRD-negative after induction. Two (7.1%) patients received allogeneic SCT. The median OS in the venetoclax cohort was 354 days (11.6 months), though no statistically significant difference was detected when compared to the intensive cohort (p = 0.0735). However, when the two cohorts were selected for adverse cytogenetic risk and compared, the median OS was not reached in the intensive group at a median follow-up of 636 days but was 91 days in the venetoclax group (p = 0.025).
Conclusion: Elderly patients had superior responses and survival to intensive induction when compared to venetoclax-based strategies when analyzed with respect to cytogenetic risk. Additionally, though the rates of toxicities were higher in the intensive cohort, the mortality during induction appeared to be lower, but did not reach statistical significance. Our work indicates that careful selection of intensive induction candidates is warranted, as those who can tolerate intensive regimens may be undertreated by selecting a less-intensive therapeutic strategy.
No relevant conflicts of interest to declare.