Abstract
INTRODUCTION: Literature on acute myeloid Leukemia (AML) from low and middle income countries (LMIC) like India indicate lower uptake of intensive treatment (29%) and higher induction mortality (25%) (Philip C, BJH 2015). This is attributable to delayed presentation, higher infection burden, multi-drug resistant organism (MDRO) infections, poor nutritional status, access to care, and high costs driven by 'out-of-pocket expenditures', among other reasons.
AIM: To determine the clinical profile, cause and costs of care of patients who die during Induction therapy of newly diagnosed AML.
METHODOLOGY: This is a retrospective chart review of newly diagnosed AML patients who died during Induction therapy. Induction mortality was defined as death within 60 days from the date of initiation of therapy for newly diagnosed AML. Secondary and therapy related AML were excluded from the study. Demographic and clinical profiling and analyses of the cause of death was undertaken.
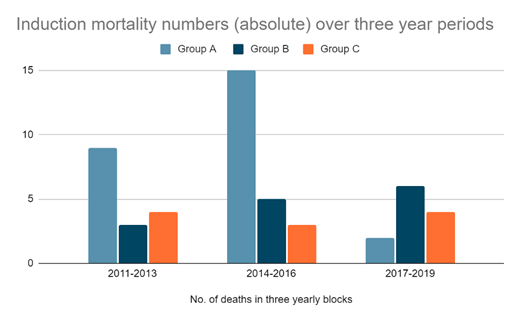
RESULTs: Between May 2011-Dec 2019, 825 adult patients were registered with newly diagnosed AML. 455 (55.2%) patients discontinued care primarily due to financial constraints, and 370 (44.8%) continued treatment here. Among these (n=370), 275 (74.3%) chose to receive standard or abbreviated intensive therapy (Group A), 58 (15.7%) chose disease control only with continuous less-intensive regimens like hypomethylating agents (Group B) and 37 (10%) chose palliation and best supportive care (Group C). Median age was 55y (20-84) overall, and 39.5y, 67.5y and 69y respectively in groups A, B and C. Females were 59% of the cohort. The overall induction mortality was 13.78% (n=51). Independently, induction mortality was 9.4% (26/275) in Group A, 24.13% (14/58) in Group B and 29.72% (11/37) in Group C, with a yearly trend suggesting a decrease in Group A (Fig 1) in the last 3 years. The most common cause of death was sepsis, 92.1% (n=47), with or without a bleeding episode. Isolated bleeding episodes were accountable for 4% (n=2) of overall deaths, and disease progression in the rest (n=2). Disease burden at presentation (% blasts in peripheral blood and marrow respectively, median values): 40% & 55% in Group A, 47% & 55% Group B, and 60% & 61% in group C. ELN 2017 risk grouping: 16% standard risk (n=8), 51% intermediate risk (n=26) & 33% high risk (n=17). 33% (n=17) had a lung infection at baseline and this was the commonest source of sepsis. Stool and throat surveillance cultures revealed MDRO (carbapenem resistant enterobacteriaceae) in 47% (n=15/32 tests) and 33% (n=7/21 tests) patients, respectively. 74.5% (n=38/51) developed blood culture positive infections during induction therapy. The median number of days to culture positivity was 11 days overall, 13 days in Group A (n=22), 3 in group B (n=13) and 1 day in Group C (n=3). The most common blood culture-detected organism was MDRO Carbapenem resistant Klebsiella (n=12). Imaging detected fungal infection was observed in 47% (n=24). The common antibiotic regimens included meropenem and colistin, with voriconazole being the most common antifungal used. The median ICU stay was 3.5 days (range of 0-24 days) in Group A and 1.5 days (range 0-13) in Group B. The median direct hospital costs in these patients were $6999 in Group A, $7923 in Group B and $2655 in Group C [per-capita GDP of India in 2019: $2099.6].
CONCLUSIONS: The induction mortality in AML varies with age and the intent of treatment. Younger patients undergoing intensive curative therapies have lesser induction mortality. Sepsis is the most common cause of induction deaths, primarily due to a high disease burden and higher infection burden at presentation, and the presence of MDRO bacteremia. Costs of care are very high and contribute to the high drop out rate from curative therapy of newly diagnosed AML.
Radhakrishnan: Novartis India: Membership on an entity's Board of Directors or advisory committees; NATCO Pharmaceuticals: Research Funding; Janssen India: Honoraria; Intas Pharmaceuticals: Research Funding; Bristol-Myers-Squibb India: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche India: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca India: Honoraria, Speakers Bureau; Aurigene: Speakers Bureau; Dr Reddy's Laboratories: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cipla Pharmaceuticals India: Research Funding; Emcure Pharmaceuticals: Research Funding. Garg: Dr Reddys Laboratories: Honoraria, Speakers Bureau. Nair: Intas pharmaceuticals: Honoraria, Speakers Bureau; Fresenius Kabi India: Honoraria; Janssen India: Honoraria, Speakers Bureau; Cipla Pharmaceuticals: Honoraria, Speakers Bureau; Novartis India: Honoraria; Dr Reddy's Laboratories: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Mylan pharmaceuticals: Honoraria. Chandy: Intas Pharmaceuticals: Research Funding; Pfizer: Honoraria; Janssen: Honoraria.