Abstract
BACKGROUND
Ibrutinib is approved as a second line treatment option for Waldenström Macroglobulinemia (WM) patients in the United Kingdom and for patients unsuitable for chemo-immunotherapy. In a clinical trial setting, Ibrutinib monotherapy produced a major response rate (MRR) of 79.4% and a predicted five-year overall survival (OS) rate of 87%1. Valuable insights can be gained on the response and tolerability outcomes of Ibrutinib monotherapy in WM outside of the clinical trial setting. This study aimed to evaluate these outcomes in a large specialist centre which receives referrals from across the UK.
METHODS
A retrospective analysis was performed on consecutive patients with WM who commenced Ibrutinib between December 2014 and February 2021; all included patients were treated for at least 3 months. Data were collated from the WMUK Rory Morrison Registry. The primary outcomes were depth of response, event free survival (EFS, defined as time from commencement to permanent ibrutinib discontinuation due to toxicity, disease progression or death), duration of response (time from best response to progressive disease, PD), progression free survival (PFS) and overall survival (OS). Response was determined in accordance with the Sixth International Workshop on WM (IWWM) response criteria 2. Baseline characteristics and toxicity data were also collated.
RESULTS
Of the 84 patients who had commenced ibrutinib, sufficient data was available for 62 patients; median age was 69 years (range 44 - 85) and 40 (64.5%) were male. Among the 50 patients for whom International Prognostic Scoring System for WM (IPSSWM) data were available, the IPSSWM risk score at the time of ibrutinib initiation was low (n=8, 16%), intermediate (n=25, 50%) and high (n=17, 34%). The median number of prior therapy lines was 2 (range 1 - 7). The median time from diagnosis to commencement of ibrutinib was 70 months (Range 2 - 243).
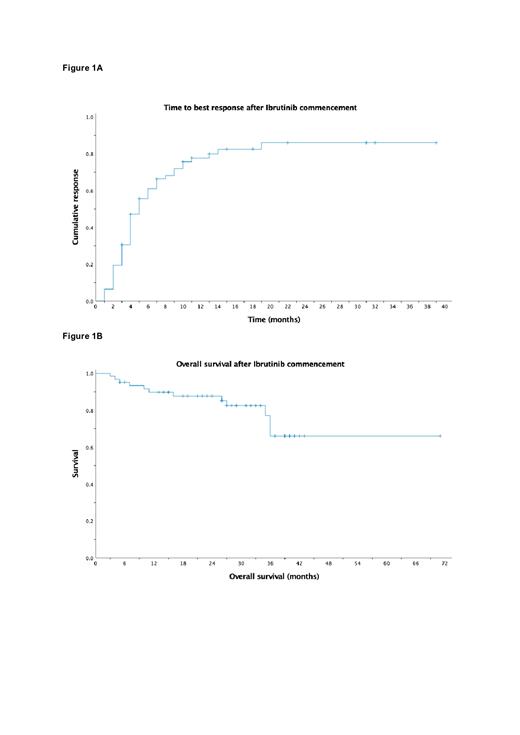
The median follow-up was 29 months (Range 3 - 71). Objective response rate (≥MR) was 79% (n=49) and MRR was 62.9% (n=39). Best response achieved was CR in 12.9%, VGPR in 24.2%, PR in 25.8%, MR in 16.1% and SD in 11.2%; 9.7% of patients experienced PD. The median time to best response was 5 months (Figure 1A) and the median EFS was 37 months (Range 3 - 71). Of the 49 patients who achieved an objective response, the median PFS was not reached and the two-year predicted PFS rate was 73.5%. At the time of analysis, 15 patients (24.2%) had discontinued ibrutinib due to disease progression, 5 (8.1%) due to adverse effects and 4 (6.5%) due to death. The median OS was not reached, and the predicted two-year OS rate was 87.8% (Figure 1B).
The most common adverse effects were diarrhoea (14.5%), atrial fibrillation (AF, 9.7%) and rash (9.7%). Six of the seven patients who developed treatment-emergent AF continued ibrutinib with concurrent anticoagulants and either rate or rhythm control. Ibrutinib was permanently discontinued due to heart failure/AF (n=1), intracranial haemorrhage whilst taking ibrutinib and warfarin concurrently for brittle antiphospholipid syndrome and WM (n=1), significant liver function derangement (n=2) and intractable diarrhoea (n=1). Ibrutinib dose was reduced to 280 mg daily (n=13) and 140 mg daily (n=4) due to adverse reactions.
CONCLUSION
In a real-world setting, ibrutinib produces clinically meaningful objective responses in patients with relapsed WM; these response rates are comparable to those achieved in a clinical trial setting. Ibrutinib can produce durable disease control with long remission intervals. The toxicity profile is acceptable.
REFERENCES
1. Treon, S., Meid, K., Gustine, J., Yang, G., Xu, L., & Liu, X. et al. (2021). Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients With Waldenström Macroglobulinemia. Journal Of Clinical Oncology, 39(6), 565-575. doi: 10.1200/jco.20.00555
2. Owen, S., Kyle, R., Stone, M., Rawstron, A., Leblond, V., Merlini, G. et al. (2013). Response assessment in Waldenström Macroglobulinemia: updated from the VIth International Workshop. British Journal of Haematology, 60, 171-176. doi: 10.1111/bjh.12102
D'Sa: Sanofi: Honoraria; BeiGene: Honoraria, Research Funding; Janssen Cilag: Honoraria, Research Funding.