Abstract
Background
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma subtype seen in Caucasian countries. Recent data were published on FL from Latin America (LATAM), presenting information of 12 countries. However, this information is widely variable because of the diversity of the Latin population (Caucasian and mestizo individuals). Our aim was to evaluate the clinical features, treatment patterns outcomes of non-Caucasian patients with FL from a single cancer center. Our second aim was to validate FLIPI1, FLIPI2, PRIMA and POD-24 score in our cohort.
Methods:
This is a retrospective study, including all patients with a pathological diagnosis of FL at the National Institute of Neoplastic Diseases in Lima, Peru from 2010 to 2019. All cases were reviewed by specialized pathologists. Baseline clinical and pathological data were collected. Responses were assessed based on the Lugano criteria. Overall survival (OS) was estimated using the Kaplan-Meier method. Differences were compared with the log-rank test.
Results
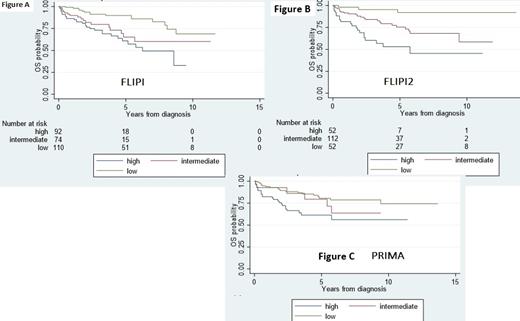
From 2010 to 2019 4480 patients with B-cell lymphoma were diagnosed, 449 patients (10%) had grade 1 to 3A FL, 302 patients had the five variables for FLIPI, 242 for FLIPI2, 257 for the 2 variables for PRIMA prognostic index and 209 received systemic treatment and had enough information to evaluate POD24. The median age for the entire cohort was 59 years old (range 24-92), 49% of patients were ≥60 years, 46% were male, 22% had extranodal involvement, 34% had bulky disease (≥6 cm in diameter), 68% had stage III/IV disease, 32% had hemoglobin <12 g/dl, 8% had serum albumin <3 g/dl, 35% had elevated serum LDH, 33% had B2-microglobulin ≥3,5 mg/l, 22% had bone marrow involvement and 23% had lymph node sites >4. Low, intermediate and high FLIPI were seen in 39%, 27% and 34% of patients, respectively. Low, intermediate and high-risk FLIPI2 was seen in 23%, 54% and 23% of patients, respectively. Low, intermediate, and high PRIMA was seen in 53%, 12% and 35%, respectively. 209 patients received systemic treatment, 60% received CHOP ± rituximab (R), 13% CVP ± R, 15% CHOP, 11% CVP. Response data were available in 158 patients with complete response in 35%, partial response in 57% and no response in 8%, for an overall response rate of 92%. 19% of patient had disease progression within 24 months of first treatment initiation (POD24). For the entire cohort, the median follow-up time was 2.6 years (Interquartile range [IQR] 0.08-13.6), the median OS was not reached (IQR 4.2-not reached). 5y was OS 72% (95% CI 65-77). 5y OS for low, intermediate and high FLIPI were 90% (95% CI 81-94.5), 65% (95% CI 47.3-78.2) and 60.1% (95% CI 46.6-72.4), respectively (p<0.001; Figure a). 5y OS for low, intermediate and high FLIPI2 were 91.9% (95% CI 76.3-97.3), 75.4% (95% CI 63.8-83.7) and 52.9% (95% CI 35.5-67.6), respectively (p<0.001; Figure b). 5y OS for low, intermediate and high PRIMA were 80.5% (95% CI 70-87.6), 79.4% (95% CI 52-92), and 61% (95% CI 47-73), respectively (p=0.004; Figure c). Patients who had and did not have POD24 had median OS of 5.7 years (IQR 2-NR) and NR (IQR 5.1-NR), respectively (p<0.001). 5y OS for patients who had and did not have POD24 was 54.1 % (95% CI 31-63) and 75.2% (95% CI 66-82), respectively (p=0.01).
Conclusion:
FL has a lower incidence in non-Caucasian patients in Peru compared to those reported for Western countries or other LATAM Caucasian population. FL patients showed higher rates of high FLIPI and FLIPI2 than previously reported in the literature. Chemoimmunotherapy is the standard approach to FL patients, which is associated with high rates of overall response, but low rate of complete response. The OS rates are shorter than those reported in the previously LATAM cohort. Our study validates the prognostic value of FLIPI1, FLIPI2, PRIMA and POD24.
No relevant conflicts of interest to declare.