Abstract
Background: Up to one-third of patients with Hodgkin lymphoma (HL) are not responsive to first-line therapy or eventually relapse. New drugs, including anti-PDL1 (i.e., nivolumab) and monoclonal antibodies (i.e., brentuximab vedotin (BV)), have changed the landscape in the treatment of HL. However, the financial toxicity associated with these new drugs is worrisome and are practically inaccessible to most patients living in low-middle income countries. The approved dose of nivolumab in refractory classical Hodgkin Lymphoma (HL) is 3 mg/kg, but lower doses have demonstrated efficacy.
Objective: To assess the efficacy of low-dose nivolumab at a fixed dose of 40 mg, 100 mg, or 140 mg in combination with BV 1.8 mg/kg every three weeks in patients with relapsed or refractory (R/R) HL.
Methods: We conducted a retrospective analysis of adults with R/R HL who received BV at 1.8 mg/kg and low-dose nivolumab at a fixed dose of 40 mg, 100 mg, or 140 mg IV every three weeks. Patients were routinely offered the highest dose possible that could be afforded for at least 4 cycles, as treatment costs were covered out of pocket. We calculated treatment responses, and overall survival (OS), and progression-free survival (PFS) with the Kaplan-Meier method.
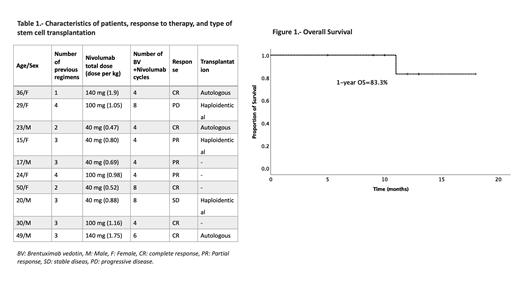
Results: A total of 10 patients (50% male, median of age 26.5 years (15-50)) with R/R HL were included; n=7 relapsed and n=3 refractory patients. Median follow-up was 11.5 months (range, 5-18) (antes o después del BV/nivo?). The median previous lines of treatment were three (range, 1-4) and regimens were ESHAP (2, 20%), IGEV (3, 30%)), CGEV (1, 10%), ICE (3, 30%), and allogenic transplantation (1, 10%). Six patients (60%) had received radiotherapy (median dose: 21.5 Gy, range: 20-25). Five patients (50%) received 40 mg of nivolumab, n=3 100 mg (30%) and n=2 140mg (20%). The median dose per cycle was 0.93 mg/kg (range, 0.48-1.9 mg/kg). After a median of 4 (range, 4-8) cycles of low-dose nivolumab + BV the overall response rate was 80% (n=8); five obtained a complete response (CR) (50%) and n=3 a partial response (30%) . One patient (10%) had stable disease, and the other progressed (10%). Grade 1 or 2 adverse events occurred in n=6 patients (60%), including neuropathy (n=2), grade 1 leucopenia (n=3), fever (n=1), and myalgia (n=1). No grade III or IV adverse events were reported. One-year OS and PFS was 83.3%. Transplant consolidation was conducted in six cases (60%) (three autologous and three haploidentical), three are awaiting transplantation and one relapsed after achieving CR before transplant conditioning. One patient died on day +21 after haploidentical transplantation in the context of progressive disease.
Conclusion: A short course of low-dose nivolumab and BV showed remarkable efficacy, achieving response in most patients with minimal toxicity and allowed patients with R/R HL who could not afford standard doses to undergo transplantation. The real-world safety and efficacy of low-dose nivolumab and BV should be further addressed in more extensive prospective studies.
Gomez-De Leon: ASH: Research Funding; Sanofi: Honoraria; Novartis: Honoraria; Abbvie: Honoraria. Gomez-Almaguer: Janssen: Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau.