Abstract
Introduction Despite the availability of vaccination against COVID 19 for all population categories since January 2021, it is moving slowly in Russia. Patients (pts) with chronic myeloid leukemia (CML) usually lead a normal life with social interactions. In the context of the COVID 19 pandemic, we find it important to identify the factors of adherence to vaccination and clarify the concerns.
Objective: To determine the proportion of CML pts willing to consider vaccination against COVID 19, adherence factors and reasons for not vaccinating.
Materials and methods. A survey on the attitude to vaccination against COVID 19 was prospectively carried out among all pts with CML consulted at the outpatient department of National Research Center for Hematology (Moscow, Russia) who agreed to participate. The key questions included considerations for and against vaccination, socio-demographic and clinical characteristics, lifestyle, comorbidities and history of COVID 19.
Results. Within 4 months (from March 15 to July 19, 2021), 172 CML pts completed the questionnaire. CML chronic phase, advanced phase and blast crisis were in 167 (97%), 4 (2%) and 1(1%) respectively. In total, 141 (82%) pts were on therapy with 1 st, 2 nd and >3 rd therapy line in 77 (55%), 33 (23%) and 31 (22%) pts, respectively. Thirty one (18%) had no therapy: 6 (3.5%) newly diagnozed, 25 (14.5%) in a treatment-free remission. A deep and major molecular response was in 77 (45%) and 30 (17%) pts, respectively. Presence and absence of molecular response MR2 was in 20 (12%) and 45 (26%) pts respectively.
The median age of pts was 46 years (range 19-82), 75(44%) were males. Married 108 (63%), 70 (41%) lived with elderly relatives, 35 (20%) with children. A higher education was in 123 (72%) pts, 123 (72%) could not work remotely and 46 (27%) had interactions to people by work. Any comorbidity was in 89 (52%) pts, 42(24%) had >1 concomitant disease, 48 (28%) had cardiovascular diseases, 44 (26%) had an obesity. A history of COVID 19 was in 41 (24%) pts and in the close circle of 74 (43%) pts.
Vaccination was supported by 94(55%) pts (with 29 (17%) already vaccinated) and not supported by 76 (44%) pts, 2 (1%) pts did not answer. Among those supporting vaccination vs not supporting there was significantly more males (52% vs 33%, p=0,012), married pts (73% vs 49%, p<0,001) and pts with higher education (88% vs 51%, p=0,006). Other factors (age, comorbidities, obesity, profession-related features, COVID 19 in pts or their environment, living with elderly relatives or children, therapy and treatment response) were not significant. Less pts were against vaccination in June-July 2021 before the 3 rd outbreak of COVID 19 compared to spring period (33% vs 50%, p=0,045).
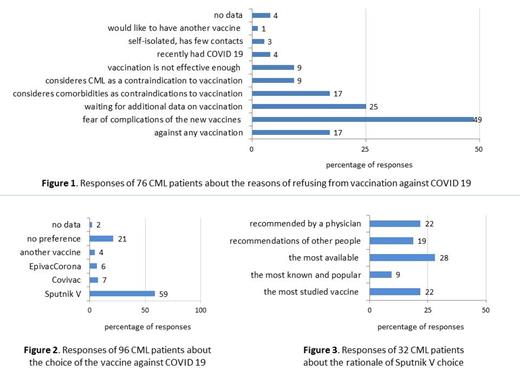
The two most common reasons to avoid vaccination were the fear of complications in 37(49%) pts and waiting for additional data in 19(25%) (Fig.1). Notably, 7 (9%) pts considered CML as a contraindication to vaccination. Among those supporting vaccination, 55(59%) preferred to choose the GamCovidVac (Sputnik V) vaccine, 20(21%) had no preference (Fig.2). Out of 32 pts who gave the rationale for the Sputnik V choice 19(59%) noted its best availability, study or popularity (Fig.3). Among 23 pts with additional questions 12 (52%) wondered about the possibility of vaccination with CML diagnosis and 6 (26%) asked help with a vaccine choice.
Conclusion: Despite access to vaccines against COVID 19 with proven efficacy and safety, almost half of CML pts (44%) do not support vaccination. Socio-demographic factors such as gender, education, marriage status appeared to be significant for this decision. Considering the frequent concerns of the possibility of vaccination with CML diagnosis as well as the fear of complications, hematologists should provide a relevant clarifying information on these issues.
Chelysheva: Pfizer: Speakers Bureau; Novartis Pharma: Speakers Bureau; Pharmstandart: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau. Petrova: Pfizer: Speakers Bureau; Novartis Pharma: Speakers Bureau. Gurianova: Pfizer: Speakers Bureau. Kokhno: Novartis Pharma: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau. Turkina: Novartis Pharma: Speakers Bureau; Pfizer: Speakers Bureau; Pharmstandart: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau.