Abstract
Introduction
Chronic myeloid leukemia is a myeloproliferative neoplasm (MPN) that is characterized by the uncontrolled production of mature and maturing granulocytes, mainly neutrophils. The molecular defect in CML is related to the reciprocal translocation between chromosomes 9 and 22, resulting in the BCR-ABL1 fusion gene. Typically, CML is BCR-ABL1 positive. Mutation like JAK2, which is seen in BCR-ABL1 negative MPN is usually absent in CML. However, there is an uncommon entity of CML which has been reported to have both BCR-ABL1 positive and JAK2 mutation concomitantly. Little is known about the characteristics of the best treatment modalities and the outcome of CML with double-positive ( JAK2 and BCR-ABL1).
Methods
We searched the English literature (Google Scholar, PubMed, and SCOPUS) for studies, reviews, case series, and case reports of patients with chronic myeloid leukemia who had JAK2 mutation. Inclusion criteria: were the presence of JAK2 mutation in CML patients with BCR-ABL1 rearrangement and, secondly, age ≥18yrs. The search included all articles published up to 20th April 2021.
Results and Discussion
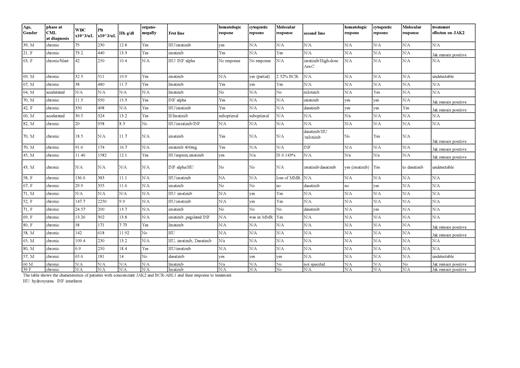
A total of 25 patients met our criteria of search. Twenty-two patients were diagnosed in the chronic phase, 2 patients in the accelerated phase, and one patient transformed to the blast phase. More females n=16 and 10 males. The mean age at the time of diagnosis was 61.3 years. 9 patients had to be switch to a second-line therapy. 7 patients were treated with imatinib; only 1 patient has loss of JAK2 mutation with imatinib (1 out of 7). While 3 patients treated with dasatinib 2 responded, and one 1 had persistent JAK2 mutation. The reason for detecting the JAK2 mutation was persistent thrombocytosis or erythrocytosis, failure to respond to treatment, or suspected other MPN. Hematological parameters appear close to other CML; patients with mean WBC 56.8 x10^3/uL, mean PLT of 541.9 x10^3/uL, and mean Hb of 12.4 g/dl. The characteristics of age and gender for this category of patients with CML are not significantly different from other CML patients. Age and gender distribution and the presence of splenomegaly or organomegaly are almost the same. Males were slightly more than females as CML has a slight male predominance. The mean age was 58 years, which is close to the mean age of CML patients.
From the data available, most patients treated with dasatinib had the JAK2 mutation undetectable on follow-up, while patients on imatinib JAK2 mutation persisted despite that some had CML in remission. This might means that the driving mutation is probably BCR-ABL. Regarding the response to the TKI 3 patients who had dasatinib 2 had a loss of JAK2 and one JAK2 persisted. Only one patient on imatinib had a loss of JAK2 out of 7. The reason behind the high success rate of achieving remission with medication like dasatinib could be explained by the drug's dual tyrosine kinase inhibitors activity. Imatinib acts by inhibiting the BCR-ABL1 pathway, inhibiting the tyrosine kinase by competitive inhibition, blocking the ATP binding site, and thereby preventing the conformational switch to the active form [0]. In comparison, a broad-spectrum kinase inhibitors (less selective) like dasatinib is (a second-generation tyrosine kinase inhibitor) inhibiting the BCR-ABL1 like imatinib, and additionally, they inhibit other signaling pathways like SRC kinases. This may allow multikinase inhibitors to have additional therapeutic effects that can include the JAK2 mutation. Patients with BCR-ABL-JAK2 Positive Chronic Myeloid Leukemiahave the same characteristics of BCR-ABL CML, and also they share some features like persistence of thrombocytosis.
Conclusion
It is difficult to conclude that multi-kinase inhibitors are superior to imatinib in treating CML with concomitant JAK2 mutation. But the result of the reported cases showed that multi-kinase inhibitors are more likely to be successful in achieving remission and loss of JAK2 mutation. However, it is difficult to generalize the result without further studies due to the few numbers of patients and the unusual association.
No relevant conflicts of interest to declare.