Abstract
Background: Since the identification of JAK2 mutations in polycythemia vera (PV) in 2005 (Kralovics et al., NEJM 2005), molecular testing of JAK2 in patients with erythrocytosis has become part of routine clinical practice. We hypothesized that changes in the World Health Organization (WHO) diagnostic criteria for PV in 2016, which lowered the hemoglobin threshold to >165 g/L for men and >160 g/L for women, may have resulted in increased molecular testing. This study examines changing patterns of utilization of molecular diagnostics in patients referred for erythrocytosis at a tertiary care center.
Methods: We examined all patients with erythrocytosis who underwent JAK2 testing, which included testing for JAK2 V617F with PCR between 2015 and 2017, and JAK2 V617F and exon 12 mutations with Next-Generation Sequencing (NGS) between 2018 and 2020 at London Health Sciences Centre in Ontario, Canada. We performed a retrospective chart review to extract laboratory and clinical data, including information on medical comorbidities and medications, with a focus on known secondary causes of erythrocytosis.
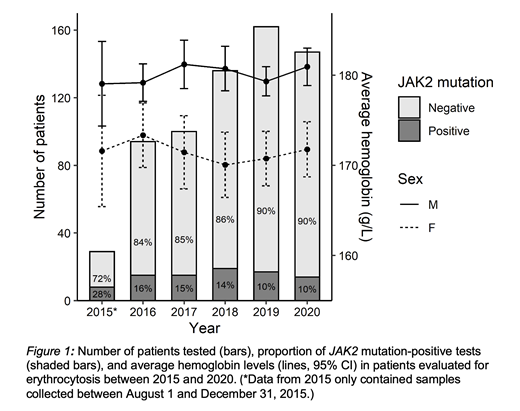
Results: A total of 668 patients with erythrocytosis underwent JAK2 testing at our institution between August 1, 2015 and December 31, 2020. There was an overall increase in testing over the five-year study period, with a decline in the positive detection rate: 8/29 (28%) in 2015, 15/94 (16%) in 2016, 15/100 (15%) in 2017, 19/136 (14%) in 2018, 17/162 (10%) in 2019, and 14/147 (10%) in 2020 (Figure 1). The average hemoglobin levels in patients with erythrocytosis who underwent testing remained similar across all years (range 170-173 g/L for women, 179-181 g/L for men). In our cohort, there was a high proportion of patients with known or suspected secondary causes of erythrocytosis who underwent molecular testing. Between 2018 and 2020, 324/445 (73%) of patients who underwent molecular testing had either chronic obstructive pulmonary disease, obstructive sleep apnea, other hypoxic lung disease, smoking history, erythropoietin-secreting tumor, or potential drug-induced erythrocytosis. Specifically, we observed an increase in proportion of patients who underwent molecular testing on sodium-glucose cotransporter-2 (SGLT-2) inhibitors, a known secondary cause of erythrocytosis, with 15/136 (11%) in 2018, 17/162 (10%) in 2019, and 25/147 (17%) in 2020. In contrast, the proportion of patients on testosterone was relatively constant at 15/136 (11%) in 2018, 11/162 (6.8%) in 2019, and 11/147 (7.5%) in 2020.
Conclusion: This study revealed that a high proportion of patients with known or suspected secondary causes of erythrocytosis underwent JAK2 testing, resulting in increase in molecular testing over time and a decline in positive detection rate. In particular, we observed a number of patients on SGLT-2 inhibitors who had investigation, suggesting that this class of medications may be an underrecognized cause of drug-induced erythrocytosis (Chin-Yee et al., CMAJ 2020). Our findings underscore the importance of careful medical history and medication review to support more judicious use of molecular testing. Similarity in average hemoglobin levels across the five-year study period suggests that other factors, such as increased availability of 'routine' molecular testing, rather than changes in the WHO diagnostic criteria may explain increases in JAK2 testing. Our study indicates a need to develop an effective clinical prediction rule for JAK2 positivity to better risk stratify patients with suspected PV based on clinical and laboratory parameters to optimize utilization of molecular diagnostics.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract