Abstract
Background: Immune dysregulation and somatic gene mutations are known prognostics factors in myelodysplastic syndromes (MDS). Moreover, impaired cytotoxicity and a decrease in mature natural killer cells (NK) have been related to higher risk characteristics. Also, killer immunoglobulin-like receptor (KIR) expression and haplotype have been associated with overall survival in MDS. The overexpression of inflammatory cytokines, produced by the clonal cell, plays a role in the immune environment. Early MDS presented increase apoptosis, whereas high risk MDS shows a downregulation of pro-apoptotic cytokines indicating decreased immune surveillance. The importance of the interaction of the immune populations and the malignant clone is not entirely understood. The aim of this study was to characterize how the microenvironment regulates the malignant clone and to describe the different immune landscape in MDS bone marrow.
Methods: We prospectively studied 50 MDS patients, 12 idiopathic cytopenia of unknown significance (ICUS) and 4 healthy donors (HD). We analyzed different immune cells in bone marrow: NK (CD3CD56+CD16+/CD56+CD16-/CD56-CD16+) and their activating (NKp46, NKp30, NKG2C, NKG2D, NKp44, DNAM) and inhibitory receptors (TIGIT, NKG2A, Irp60, and PD1) as well as their ligands (HLA-ABC, MICA-B, CD155, PD-L1). We also assessed myeloid-derived suppressor cells (MDSC), differentiating granulocytic (Gr-MDSC: CD11b +CD33 +HLA-DR -CD15 +CD14 -) from monocytic (Mo-MDSC: CD11b +CD33 +HLA-DRlow/CD15 +CD14 +). Also, T cells subpopulations in peripheral blood with the following markers (CD3/CD4/ CD8/CCR7/CD45RA/ CD27/CD28/CD279/CD57/CXCR3/CCR6). Molecular analysis by NGS using the Oncomine Myeloid Research Assay (ThermoFisher Scientific) included 40 genes associated with myeloid malignancies. Also, we determined the KIR haplotype by NGS. For the study of cytokines concentrations, we used the Luminex® platform with ThermoFisher commercial kit ProcartaPlex TM Multiplex immunoassay.
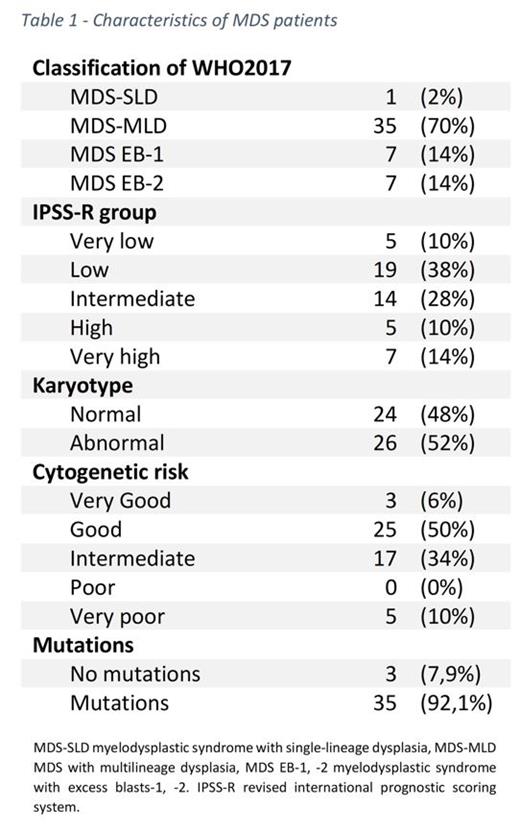
Results: A total of 66 samples were tested. Patient's median age was 74 years-old and 44% were female (other details in table1). Compared to ICUS, we found in MDS patients a decrease of TCD4+PD1+ T cells (MDS 26.23% vs ICUS 41.23%, p=0.022), effector TCD8+ cells (MDS 15.74% vs ICUS 45.69%, p= 0.02) and in TNK (MDS 1.72% vs ICUS 7.8%, p= 0.04). Regarding NK cells, we observed a decrease in mature NK (CD56dimCD16+) in MDS compared to ICUS, which did not reach statistical significance (MDS 15.25% vs ICUS 79.16%; p=0.104). As for NK receptors, we observed a significant decrease in NKG2C (MDS 4.94%, vs HD 28.35% p=0.039) and KIR2DS4 (MDS 16.56% vs HD 91.18%; p=0.036) expression in MDS. In the study of ligands, a significant loss of MIC-A/B in MDS vs. controls (MDS 0.42% vs HD 6.96%, p=0.034) was detected. Regarding cytotoxicity, a higher expression of perforin in MDS and ICUS compared with HD (35%, 42.65% vs. 11.93% respectively; p=0.033) was showed. A 33% of patients presented with KIR A haplotype, with no differences in the immunological profile between haplotypes. In terms of MDSC, we observed a trend to higher expression in MDS compared to controls (MDS 1.58%, ICUS 0.15% vs HD 0.18% p=0.10). Of these patients, 4 required treatment and 1 progressed to AML. We found mutations in 34 (85%) of MDS, of these, 27 (79.4%) had more than 2, with 38% of patients with abnormal cytogenetic (including 14.7% complex karyotype). Mutated patients had more MDSC than unmutated patients (0.95% vs 0.01%, p=0.001) and a trend to lower CD56dimCD16+ expression in mutated patients compared with unmutated MDS (24.7% vs. 91.57%, respectively, p=0.058). Finally, in the cytokines analysis, an increased level of IL-10 in high-risk compared to low and intermediate patients (2 pg/ml vs. 1 pg/ml, p=0.04) was demonstrated, 16 (53%) had high concentrations of IL10 > 40 pg/ml, 8 (26.6%) had more than 2 mutations and 3 (10%) had a single TP53 mutation.
Conclusions: Our analysis showed a heterogeneous distribution of the different immune populations. We found a decreased mature NK and increased MDSC in mutated patients. Further analyses should be performed to describe independent factors that may affect disease progression.
Molero Yordi: Oryzon Genomics: Consultancy. Salamero: Pfizer: Consultancy; BMS/Celgene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Diez-Campelo: Takeda Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Prosper: Oryzon: Honoraria; BMS-Celgene: Honoraria, Research Funding; Janssen: Honoraria. Bosch: Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Other: Travel. Valcarcel: SANOFI: Consultancy, Honoraria, Speakers Bureau; SOBI: Consultancy, Honoraria, Speakers Bureau; JAZZ: Consultancy, Honoraria, Speakers Bureau; AMGEN: Consultancy, Honoraria, Speakers Bureau; NOVARTIS: Consultancy, Honoraria, Speakers Bureau; ASTELLAS: Consultancy, Honoraria, Speakers Bureau; TAKEDA: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; CELGENE: Consultancy, Honoraria, Speakers Bureau.