Abstract
Introduction
Myelodysplastic syndromes (MDS), a heterogeneous group of acquired clonal hematopoietic stem cell disorders, is characterized by peripheral cytopenia and has a high tendency to progress to acute myeloid leukemia (AML). Serum ferritin (SF) level is a well-known indicator of iron stores and inflammation. Elevated SF levels is common in patients with MDS and appear to be largely related to regular red blood cell (RBC) transfusions. However, some patients with MDS showed high SF levels at the time of diagnosis, even before receiving RBC transfusions, although the exact mechanisms is unclear. The impact of SF elevation without RBC transfusion has not been well characterized. In this study, we assessed cytogenetic, molecular, and clinical characteristics of patients with MDS and SF elevation.
Methods
Patients with newly diagnosed MDS who visited our hospital between Jan 2011 and Jan 2020 were included in this study. Patients were defined as high-SF-MDS when SF values were >350 ng/mL (patients with <350 ng/mL were defined as low-SF-MDS). Patients with autoimmune diseases, active infections, hemophagocytic syndromes, and RBC transfusion were excluded. Baseline characteristics were compared using Fisher's exact test for categorical variables and the Mann-Whitney test for continuous variables. The log-rank test was used for the overall survival (OS) analysis. The cumulative incidence of AML progression was estimated and compared using Gray's method. In next-generation sequencing (NGS) analysis, 68 genes associated with myeloid malignancy were analyzed using DNA extracted from bone marrow samples.
Results
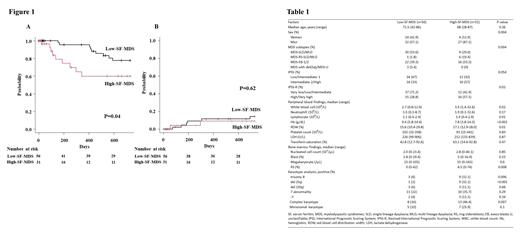
Among the 112 patients with MDS, SF value were available for 87 patients. This study analyzed 28 women and 59 men with a median age of 69 years (range: 28-87 years). The median follow-up period was 540 days (range: 3-3618 days). A total of 17 patients died during the observational period (until Apr 2021). Patients were diagnosed with MDS with single lineage dysplasia (MDS-SLD, n = 9), MDS with multilineage dysplasia (MDS-MLD, n = 26), MDS with ring sideroblasts with SLD (MDS-RS-SLD, n = 5), MDS-RS-MLD (n = 6), MDS with excess blasts 1 (MDS-EB1, n = 25), MDS-EB2 (n = 13), MDS with isolated del(5q) (n=1), and MDS, unclassifiable (MDS-U, n = 2). The median SF value was 263 ng/mL (range: 3-1,245 ng/mL); 31 patients were classified as high-SF-MDS and 56 were classified as low-SF-MDS. The Proportion of MDS subtypes differed significantly between the groups (P=0.004, Table1). Compared to low-SF-MDS, MDS-EB1/2 (51.6% vs. 39.3%) and MDS-RS-SLD/MLD (22.6% vs. 1.8%) was more common in high-SF-MDS. In the laboratory data analysis, high-SF-MDS patients showed low Hb levels, high RBC distribution width values, and high bone marrow RS percentages. In patients with high-SF-MDS, as well as MDS-RS, the presence of bone marrow RS was also observed in seven out of 11 patients with MDS-EB1/2 (median 8%, range 2-19%). Complex karyotype ( was more common in patients with high-SF-MDS (46.4% vs. 16.0%, P=0.007) than those with low-SF-MDS. Trisomy 8 and del (5q) were also frequently detected among those with high-SF-MDS. Patients with high-SF-MDS had worse 2-year overall survival (OS) than those with low-SF-MDS. To clarify the molecular characteristics of high-SF-MDS in more detail, NGS analysis was performed on 11 patients with high-SF-MDS whose samples were available. Ten gene mutations were detected in eight patients. Interestingly, five among 11 patients harbored TP53 mutation; the median variant allele frequency was 53.9% (range, 39.7-64.1%). Subsequently, SF3B1 gene mutation was detected in three patients; and two of them also harbored TP53 mutation. Other gene mutations, RUNX1, STAG2, SRSF2, ASXL1, ATM, DNMT3A, BCOR, and DDX41 were detected in one patient each.
No relevant conflicts of interest to declare.