Abstract
Background:
Chronic lymphocytic leukemia (CLL) is the most common leukemia among adults in the United States. Despite the advancements in the treatment of CLL, relapsed/refractory CLL (RR-CLL) cases are still associated with poor prognosis. Anti-CD19 chimeric antigen receptor T cell (CAR-T) therapy has shown promising initial responses in patients with RR-CLL. We present a systematic review and meta-analysis to investigate the outcomes with CAR-T cell therapy in RR-CLL patients.
Methods:
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, 196 articles were searched from 3 databases (PubMed, Cochrane Register of Controlled Trials, and clinicaltrials.gov) using MeSH terms and keywords for "CLL" AND "CAR-T" and "Antigen CD19" OR "adoptive immunotherapy" from the date of inception to March 2021. Studies were screened by title, abstract, and full text. Original studies reporting adult patients with RR-CLL who received CD19 CAR-T therapy as the only intervention were included while reviews, duplicate, and non-relevant articles were excluded. A total of 5 studies (clinical trials) were included and the following outcomes were extracted: complete remission (CR), partial response (PR), overall response rate (ORR), overall survival (OS), progression-free survival (PFS), progressive disease (PD), cytokine release syndrome (CRS) and neurotoxicity (NT). The Joanna Briggs Institute (JBI) critical appraisal checklist for studies reporting prevalence data and randomized control trials was used for quality assessment, and all studies were reported as good. The variance between the studies was calculated using Der Simonian-Laird Estimator. Proportions along with a 95% confidence interval (CI) were extracted to compute pooled analysis using the 'meta' package by Schwarzer et al. in the R programming language (version 4.16-2).
Results:
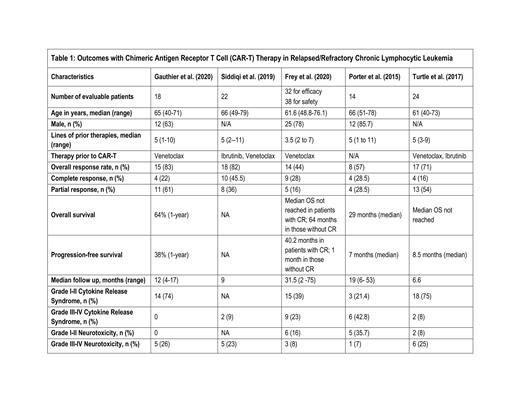
We included 137 patients from 5 studies. Out of these, a total of 110 patients were evaluated for efficacy and 116 for safety. (Table 1) Median age of the patients was 64 (40-79) years. Seventy-six percent were males as reported by 3 studies. Median number of prior therapies was 4.7 (1-11) and median follow up time was 16 (2-75) months. The pooled analysis showed a CR and PR of 28% (95 % CI 0.19-0.38, I 2=14%, n=110) and 38% (95% CI 0.21-0.57, I 2=72%, n=110) respectively with an ORR of 68% (95% CI 0.51-0.83, I 2=67%, n=110). The pooled prevalence of PD was 21% (95% CI 0.03-0.48, p= <0.01, I 2= 88%, n=110). PFS ranged from 1 month to 40.2 months as reported by 3 studies. OS ranged from 29 months to 64 months as reported by 2 studies. The pooled incidence of Grade I-II and Grade III-IV CRS was 56% (95% CI 0.32-0.79, I 2=80%, n=88) and 14% (95% CI 0.28-0.31, I 2=76%, n=110) respectively. The pooled incidence of grade I-II and grade III-IV NT was 12% (95% CI 0.15-0.29, I 2=72%, n=88) and 17.5% (95% CI 0.98-0.26, I 2=19%, n=110) respectively.
Conclusion:
CD19 CAR-T cell therapy shows favorable response rates in patients with RR-CLL who have failed multiple prior therapies with an acceptable safety profile. However, it is imperative that large prospective studies should be conducted to confirm these findings.
McGuirk: Gamida Cell: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding; Novartis: Research Funding; Bellicum Pharmaceuticals: Research Funding; Astelllas Pharma: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Pluristem Therapeutics: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy.