Abstract
The therapy of hematologic malignancies has been revolutionized by the development of therapies using chimeric antigen receptor modified T-cells (CAR-T). However, CAR-T cell therapy is often associated with cytokine release syndrome (CRS), characterized by fevers, hypotension, and hypoxia, as well as immune effector cell-associated neurotoxicity syndrome (ICANS). Severe cases of CRS can result in significant morbidity and mortality. Although disease features such as tumor burden may predict for more severe CRS and ICANS, other clinical features and biomarkers predictive of CRS and ICANS remain lacking, with the exception of measurements of serum cytokines following cell infusion (PMID: 27076371, PMID: 24553386). Experimental models have implicated elaboration of inflammatory cytokines such as IL-1 and IL-6 by monocytes in the pathogenesis of CRS and ICANS (PMID: 29808007, PMID: 29808005). Accordingly, the severity and duration of CRS and ICANS can be mitigated in part by IL-6 receptor blockade with tocilizumab and treatment with corticosteroids such as dexamethasone. Recent work has implicated ascorbate in the regulation of the activity of TET enzymes in hematopoietic cells (PMID: 28825709, PMID: 28823558). Given that TET2 deficiency has been associated with increased elaboration of inflammatory cytokines such as IL-6 and IL-1 by macrophages (PMID 3026882, PMID: 28104796, PMID: 28636844), we reasoned that ascorbate deficiency might predict for more pronounced cytokine release in patients leading to more severe CRS or ICANS.
We identified 13 patients receiving CAR-T cell therapy for hematologic malignancies at the University of Texas Southwestern. Plasma specimens were collected from patients at baseline prior to receipt of lymphodepleting chemotherapy and/or at two weeks following CAR-T cell infusion. Given the poor reliability of clinical ascorbate measurements due to oxidation, we used an optimized protocol incorporating a C13-labeled ascorbate internal standard to obtain highly precise serum measurements using liquid-chromatography mass spectrometry. The incidence and severity of CRS and ICANS was classified using standardized grading criteria as per the American Society for Transplantation and Cellular Therapy.
We measured serum ascorbate in 7 baseline and 12 post CAR-T cell infusion specimens obtained from 13 patients, with a median age of 65 (range 53 to 77). The cohort included eight patients with diffuse large B-cell lymphoma and two patients with mantle cell lymphoma receiving CD19-targeted CAR-T cells, as well as three patients with multiple myeloma receiving BCMA-targeted CAR-T cells. Eight patients developed grade one CRS, three patients developed grade two CRS, and two patients did not develop CRS. One patient developed grade one ICANS, one developed grade two ICANS, and one developed grade three ICANS. Eight patients received dexamethasone for CRS or ICANS, and eight patients received tocilizumab. Five patients only received one dose of tocilizumab, while two received two doses and one received three doses.
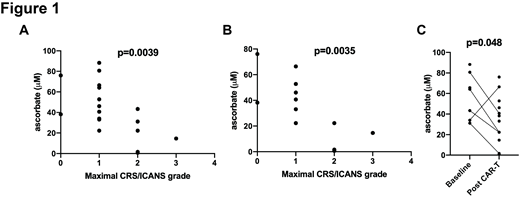
Taking all pre- and post-CAR-T cell infusion ascorbate measurements into account, a significant correlation was found between having low serum ascorbate levels and a higher maximal grade of CRS or ICANS (Figure 1A, r 2=-0.64, p=0.0039). Post-infusion ascorbate measurements also demonstrated a significant correlation between low serum ascorbate levels and higher maximal CRS or ICANS (Figure 1B, r 2=-0.78, p=0.0035), while there was no correlation between pre-infusion ascorbate measurements and CRS or ICANS. Finally, we noted a significant decrease in serum ascorbate levels when comparing pre-infusion to post-infusion specimens (Figure 1C, p=0.048), including five paired specimens. There was no significant correlation between serum ascorbate levels and the number of doses of tocilizumab or dexamethasone administered.
Low serum ascorbate levels may be associated with an increased risk for developing severe CRS and ICANS following CAR-T cell therapy. Although follow-up studies with a larger cohort of patient are necessary to substantiate this correlation, these data provide preliminary evidence that serum ascorbate levels may serve as a useful biomarker to predict severity of CRS and ICANS. Furthermore, they suggest ascorbate supplementation as a promising future strategy to mitigate these common complications of CAR-T cell therapy.
Kansagra: Alynylam, Celgene/BMS, Cota Health, GSK, Janssen, Karyopharm, Oncopeptide, Pfizer, Takeda, Sanofi: Membership on an entity's Board of Directors or advisory committees. Anderson: Celgene, BMS, Janssen, GSK, Karyopharm, Oncopeptides, Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Awan: Abbvie: Consultancy; Dava Oncology: Consultancy; Johnson and Johnson: Consultancy; Incyte: Consultancy; BMS: Consultancy; Astrazeneca: Consultancy; ADCT therapeutics: Consultancy; Pharmacyclics: Consultancy; Janssen: Consultancy; Beigene: Consultancy; Merck: Consultancy; Gilead sciences: Consultancy; Cardinal Health: Consultancy; Verastem: Consultancy; MEI Pharma: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Kite pharma: Consultancy; Genentech: Consultancy. Madanat: Stem line pharmaceutical: Honoraria; Blue Print Pharmaceutical: Honoraria; Onc Live: Honoraria; Geron Pharmaceutical: Consultancy. Patel: Agios: Membership on an entity's Board of Directors or advisory committees; PVI: Honoraria; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees. Sweetenham: EMA Wellness: Membership on an entity's Board of Directors or advisory committees.