Abstract
Intro: Acute and chronic graft versus host disease (GVHD) is a major complication of allogeneic hematopoietic stem cell transplant (allo-HSCT). Post-transplant cyclophosphamide (PTCy), alongside mycophenolate mofetil (MMF) and tacrolimus (TAC), is known to be effective in reducing cGVHD in haploidentical (HI) transplant recipients. Results of the prospective randomized HOVON-96 trial in recipients of matched related (MRD) and unrelated donors (MUD) showed that PTCy leads to a reduction in risk of severe cGVHD. However, the role of PTCy in HSCT with acute lymphoblastic leukemia (ALL) remains controversial. The purpose of this study is to evaluate outcomes using PTCy, TAC, and MMF for GVHD prophylaxis in MRD and MUD stem cell recipients who have ALL.
Methods: This study is a retrospective analysis of adult patients (aged 19 and up) with ALL who received MRD, MUD, and HI HSCT at USC Norris Cancer Hospital from 2018 to 2020. The primary end-points were to compare incidence of aGVHD and cGVHD within the first year post-transplant after receiving MRD, MUD, and HI donor transplants. Secondary end-points were day +100 mortality, 1-year overall survival (OS), relapse free survival (RFS), transplant-related mortality (TRM), and GRFS within the same subgroups. Kaplan-Meier curves and Log Rank tests were used to evaluate a difference in outcomes.
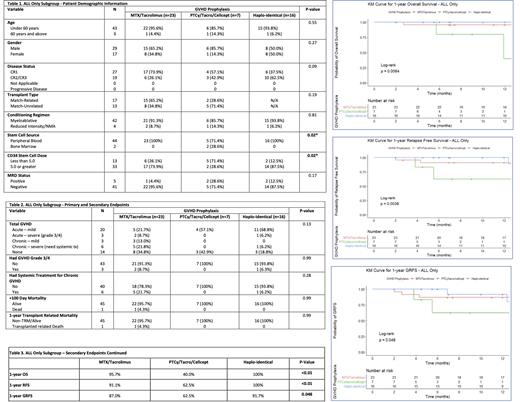
Results: A total of 46 patients with ALL who received HSCTs were identified and separated based on type of GVHD prophylaxis received: 7 (15.1%), 16 (34.8%), and 23 (50.0%) in the PT-Cy, HI and standard groups respectively. Demographic data can be found on Table 1 attached. Baseline characteristics among all groups were similar with the exception of the more Human Leukocyte Antigen (HLA) mismatch (p<0.01), higher number of patients receiving stem cells from bone marrow (p=0.02) and the increased proportion of patients receiving less than 5.0 x 10^6 CD34+ cells/kg (p=0.02) in the PTCy group. With regards to the primary end-points as seen on Table 2, there was a non-significant trend toward less frequent severe aGVHD and severe cGVHD in the PTCy group compared to the standard and HI cohorts (p=0.13). The rates of severe aGVHD were 0%, 6.2%, and 8.7% in the PT-Cy, HI and standard groups respectively. The rates of severe cGVHD were 0%, 6.2%, and 21.8% in the PT-Cy, HI and standard groups respectively. With regards to the secondary end-points in our study, as seen on Tables 2 and 3, patients receiving standard GVHD prophylaxis had a 95.7% 1-year OS, a 91.1% 1-year RFS, and 87.0% 1-year GRFS. Moreover, MRD/MUD recipients receiving PTCy for GVHD prophylaxis had worse outcomes with an 40.0% 1-year OS, a 62.5% 1-year RFS, and 62.5% 1-year GRFS. Comparatively, patients receiving HI transplants exhibited a 100% 1-year OS, a 100% 1-year RFS, and 91.7% 1-year GRFS. This was statistically significant across all outcome measures: OS (p=<0.01), RFS (p=0.0038), and GRFS (p=0.048). Kaplan-Meier curves for OS, GRFS, and RFS are attached.
Discussion: In this single institution analysis, we observed that patients with ALL who underwent HI transplant had the best outcomes. Moreover, patients receiving MUD/MRD HSCT with use of PTCy had a non-significant reduced incidence of aGVHD and cGVHD, though had significantly worse 1-year OS, RFS, and GRFS when compared to standard GVHD prophylaxis or patients receiving HI transplants. We believe this is likely a multifactorial process. However, the increased percentage of mismatched antigens in the MUD transplants (p=0.19) and higher proportion of patients receiving less than 5.0 x 10^6 CD34+ cells/kg (p=0.02) in the PTCy group is important to acknowledge and can also be contributing to their poorer outcomes. We pose the idea that the use of PTCy may also lead to a reduced graft vs. leukemia effect in this population and thus a higher incidence of relapse and worse outcomes overall. A larger, prospective study should be considered to take into account these factors to better determine if patients with ALL could benefit from PTCy for GVHD prophylaxis.
Chaudhary: Celldex: Current equity holder in publicly-traded company; Moderna: Current equity holder in publicly-traded company; Pancella: Consultancy; Oncotartis: Consultancy; Athelas: Consultancy, Current holder of stock options in a privately-held company; TCR2: Current equity holder in publicly-traded company; Allogene: Current equity holder in publicly-traded company; Angeles Therapeutics: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Founder, Patents & Royalties: Cell therapy . Yaghmour: Takeda: Consultancy, Speakers Bureau; Astellas: Speakers Bureau; Alexion: Speakers Bureau; BMS: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Agios: Consultancy, Speakers Bureau; Jazz: Speakers Bureau.