Abstract
Introduction. Severe hemophilia A (HA) negatively impacts health-related quality of life (HRQOL) through joint pain and disability, treatment burden, and effects on mental and emotional health; chronic pain and mental health are core outcomes for hemophilia gene therapy trials (Iorio, et al. Haemophilia 2018;24[4]:e167-72). In the phase 3 trial GENEr8-1 (NCT03370913), men with severe HA who received valoctocogene roxaparvovec (AAV5-hFVIII-SQ) gene therapy experienced a significant increase in endogenous FVIII activity that resulted in reduced bleeding events and FVIII utilization from baseline through 52 weeks (Ozelo, et al. Res Pract Thromb Haemost 2021;5). Here, we analyzed the impact of valoctocogene roxaparvovec on HRQOL.
Methods. Men ≥18 years of age with FVIII ≤1 IU/dL previously receiving standard-of-care FVIII prophylaxis and without history of FVIII inhibitors received one 6x10 13 vg/kg valoctocogene roxaparvovec infusion. This analysis included HIV-negative participants who completed the weeks 49-52 visit. Participants completed QOL questionnaires at baseline and weeks 4, 12, 26, and 52 post-gene therapy infusion. Change from baseline was assessed with a two-sided t-test without multiplicity control. Missing data were not imputed.
Measures included the hemophilia-specific Haemo-QOL-A questionnaire, which consists of 6 domain scores (Physical Functioning, Role Functioning, Consequences of Bleeding, Worry, Emotional Impact, Treatment Concern) and Total Score ranging from 0-100 (Rentz, et al. Haemophilia 2008;14[5]:1023-34); and the EQ-5D-5L, a general measure of HRQOL that assesses functional dimensions (Mobility, Self-Care, Usual Activities) as well as Pain/Discomfort and Anxiety/Depression (Herdman, et al. Qual Life Res 2011;20[10]:1727-36). EQ-5D-5L results are reported as visual analog scale (VAS; range, 0-100) and Utility Index Score (range, 0-1), which is calculated from population norms (Janssen, et al. Qual Life Res 2013;22[7]:171-27). Higher scores indicate better HRQOL. The anchor-based clinically important differences (CID) used for the Haemo-QOL-A were 5.5 for Total Score and 6 for domain scores (Quinn, et al. Abstract, Hemophilia Federation of America Virtual Conference, Aug 2020). For the EQ-5D-5L Index, 0.03 was considered a CID (Kaplan. COPD 2005;2[1]:91-7). However, use of general population norms, together with the presence of a disability paradox in hemophilia, causes the EQ-5D-5L to underestimate the negative impact of hemophilia on HRQOL (O'Hara, et al. Haemophilia 2021;27[2]:245-52), likely leading to underestimation of changes in HRQOL.
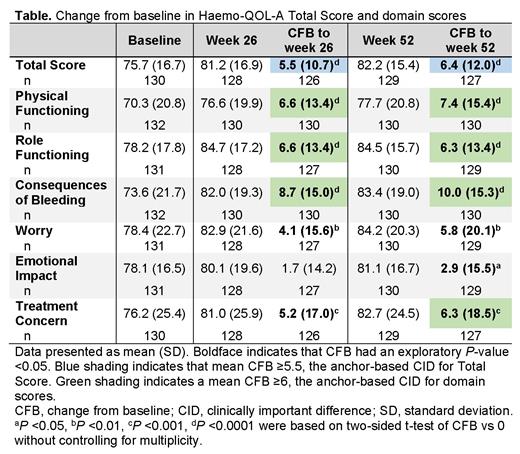
Results. This analysis included 132 participants. At baseline, mean ± standard deviation (SD) Haemo-QOL-A Total Score was 75.7 ± 16.7 (n = 130); at weeks 26 and 52, mean change from baseline in Total Score demonstrated improvement (P <0.0001) at or above the CID threshold (Table). Improvement in Total Score was noted as early as 4 weeks (change from baseline, 3.0 ± 8.5; P = 0.0001) and by week 12 had reached the CID (5.5 ± 8.4; P <0.0001). Domain scores for Physical Function, Role Functioning, and Consequences of Bleeding also improved from baseline (P <0.0001), exceeding the CID at weeks 26 and 52 (Table). Treatment Concern demonstrated improvement from baseline (P <0.001) at weeks 26 and 52, exceeding the CID at week 52. Improvements below the CID were noted for Worry at weeks 26 and 52 (P <0.01) and Emotional Impact at week 52 (P <0.05; Table).
At baseline, EQ-5D-5L VAS mean ± SD was 80.1 ± 15.3 (n = 131), which increased by 2.5 ± 13.7 (n = 129) at week 26 and 4.5 ± 13.3 (n = 129) at week 52. Mean ± SD EQ-5D-5L Index Score at baseline was 0.78 ± 0.17 (n = 131); mean ± SD changes from baseline at both week 26 (0.04 ± 0.14 [n = 128]) and 52 (0.04 ± 0.16 [n = 129]) demonstrated improvement (P ≤0.002), exceeding the CID of 0.03.
Conclusions. These data demonstrate improvement in core outcomes of mental health (EQ-5D-5L Anxiety/Depression, Haemo-QOL-A Consequences of Bleeding) and pain and discomfort (EQ-5D-5L Pain/Discomfort, Haemo-QOL-A Physical Functioning) as well as ability to perform activities of daily living (EQ-5D-5L Self-Care and Mobility, Haemo-QOL-A Physical Functioning and Role Functioning) for participants with HA following treatment with valoctocogene roxaparvovec compared with their values on standard-of-care FVIII prophylaxis.
O'Mahony: Freeline: Consultancy; Uniqure: Speakers Bureau; BioMarin Pharmaceutical Inc.: Consultancy. Mahlangu: Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Catalyst Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biomarin: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Univeristy of the Witwatersrand: Current Employment; Spark: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Unique: Research Funding; Sanofi: Research Funding, Speakers Bureau; Takeda: Speakers Bureau; WFH: Speakers Bureau; ISTH: Speakers Bureau; Springer: Speakers Bureau. Peerlinck: SOBI: Consultancy, Research Funding; Octapharma: Consultancy; NovoNordisk: Consultancy, Research Funding; Pfizer: Consultancy; Roche: Other: Clinical trial investigator, Research Funding; CSL Behring: Research Funding; Uniqure: Other: Clinical trial investigator; BIoMarin: Other: Clinical trial investigator. Lowe: Biomarin: Research Funding; Novartis: Honoraria; Sobi: Honoraria; Leo: Honoraria; Alexion: Honoraria; Takeda: Honoraria; NovoNordisk: Honoraria. Giermasz: Bayer: Consultancy; ATHN: Consultancy; NovoNordisk: Consultancy; UniQure: Consultancy, Research Funding; Sanofi Genzyme: Consultancy; Bioverativ/Sanofi: Consultancy, Research Funding, Speakers Bureau; Sangamo Therapeutics,: Research Funding; Pfizer: Consultancy; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; BioMarin: Consultancy, Research Funding. Cockrell: Takeda: Consultancy; CSL Behring: Consultancy; HEMA Biologics: Consultancy; Sanofi: Consultancy; BioMarin: Consultancy; Novo Nordisk: Consultancy; Genentech: Consultancy, Speakers Bureau. Pepperell: Pfizer: Other: Travel support. Chambost: Bayer: Consultancy; BioMarin: Consultancy, Other: Clinical trial investigator; travel support, Speakers Bureau; Roche: Consultancy, Other: Clinical trial investigator; travel support, Speakers Bureau; Sobi: Consultancy, Other: Clinical trial investigator; travel support, Speakers Bureau; Bioverativ: Other: Clinical trial investigator; CSL Behring: Other: Clinical trial investigator; travel support; LFB: Other: Clinical trial investigator; Octapharma: Other: Clinical trial investigator; travel support; Pfizer: Other: clinical trial investigator, Speakers Bureau. Majerus: BioMarin Pharmaceutical Inc.: Consultancy, Other: Clinical trial investigator, Travel support. Skinner: Bayer: Consultancy; BioMarin: Consultancy, Research Funding; Pfizer (DMC): Consultancy; Roche/Genentech: Consultancy, Research Funding; Sanofi: Consultancy; Spark (DMC): Consultancy; Takeda: Consultancy, Research Funding; Freeline: Research Funding; uniQure: Research Funding. Klamroth: Bayer: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Pfizer: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Shire (a Takeda company): Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; BioMarin: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Biotest: Consultancy, Other: Travel support, Speakers Bureau; Roche/Cugai: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Octapharma: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Sanofi: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Sobi: Consultancy, Other, Speakers Bureau; Uniqure: Consultancy, Other; LEO: Other, Research Funding, Speakers Bureau; Daiichi Sankyo: Other, Speakers Bureau; Grifols: Speakers Bureau. Quinn: BioMarin Pharmaceutical: Current Employment, Current equity holder in publicly-traded company. Yu: BioMarin Pharmaceutical: Current Employment, Current equity holder in publicly-traded company. Wong: BioMarin Pharmaceutical Inc.: Current Employment, Current equity holder in publicly-traded company. Lawal: BioMarin Pharmaceutical Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Robinson: BioMarin Pharmaceutical Inc.: Current Employment, Current equity holder in publicly-traded company. Kim: BioMarin Pharmaceutical Inc.: Current Employment, Current equity holder in publicly-traded company.