Abstract
Introduction: Hypomethylating agents (HMAs) are standard of care treatment for patients with higher-risk myelodysplastic syndromes (HR-MDS) who are ineligible for stem-cell transplantation or intensive chemotherapy. Until recently, approved HMAs included intravenous or subcutaneous azacitidine and decitabine, which should be administered for a minimum of 4-6 cycles to elicit response in the absence of progression or unacceptable toxicity. In real-world clinical practice, underutilization of HMA therapy has been documented; however, prior estimates have utilized data preceding 2016. The study objectives were to understand recent treatment utilization and characteristics among patients newly diagnosed with HR-MDS in the United States (US).
Methods: This retrospective observational study utilized the IQVIA PharMetrics ® Plus database which comprises adjudicated claims for more than 190 million unique patients across the US. Adult patients newly diagnosed with HR-MDS from July 1, 2016 through December 31, 2019 were included. Patients had ≥1 inpatient claim or ≥2 outpatient claims >60 days apart with diagnosis codes for high grade MDS lesions and/or refractory anemia with excess blasts (RAEB), and continuous enrollment for 6 months prior to and ≥180 days following the index date (index date = first diagnosis claim). Patients had a variable follow-up period up to 1 year post-index, and were followed through June 30, 2020. Patient characteristics at baseline, and treatment utilization based on observed MDS-related therapy (HMAs and supportive care), over the variable follow-up period were evaluated.
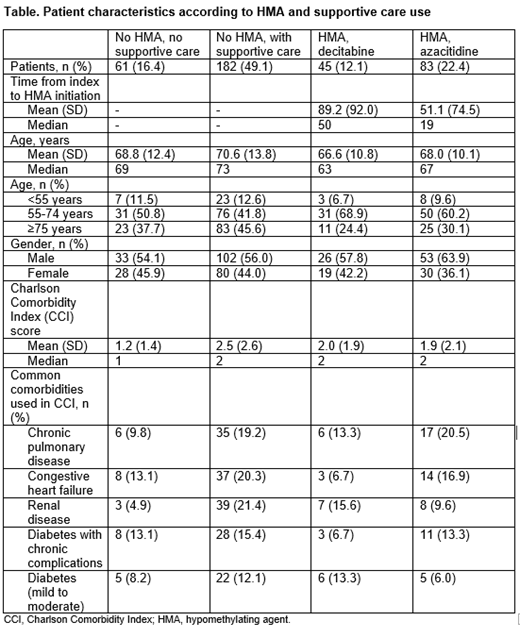
Results: A total of 371 patients were included; the mean age of patients at baseline was 69.2 years and 57.7% were male. Of these, 61 (16.4%) patients received no HMA and no supportive care over the variable follow-up period following diagnosis. Half (n=182, 49.1%) received no HMA but received supportive care, and the remainder (n=128, 34.5%) received HMA therapy over the variable follow-up period; 45 (12.1%) received decitabine as the first HMA in a mean of 89.2 days from diagnosis, and 83 (22.4%) received azacitidine as the first HMA in a mean of 51.1 days from diagnosis (Table). Patients receiving no HMA therapy but with supportive care were older (mean age 70.6 years; 45.6% aged ≥75 years) and had higher comorbidity burden (mean Charlson Comorbidity Index [CCI] 2.5) than the other cohorts (mean age 66.6-68.8 years; 24.4-37.7% aged ≥75 years; CCI 1.2-2.0). For example, a higher proportion of patients receiving no HMA therapy but with supportive care had baseline renal disease (21.4%) compared to the other cohorts (4.9-15.6%). Patients receiving no HMA or supportive care in this study had a similar mean age compared to those who received HMAs (68.8 vs 66.6-68.0 years), but tended to have lower comorbidity burden (CCI 1.2 vs 1.9-2.0).
Conclusions: In this real-world study using contemporary data, utilization of HMA therapy among patients with HR-MDS in the US was low, with only around one-third of patients receiving HMAs. Patients who did not receive HMAs but received supportive care tended to be older and have higher comorbidity burden than those who received HMAs, suggesting potential barriers for physicians in prescribing HMAs to older, frailer patients.
Zeidan: Pfizer: Other: Travel support, Research Funding; Jazz: Consultancy; Jasper: Consultancy; AstraZeneca: Consultancy; Janssen: Consultancy; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Ionis: Consultancy; Kura: Consultancy, Other: Clinical Trial Committees; Incyte: Consultancy, Research Funding; Gilead: Consultancy, Other: Clinical Trial Committees; Genentech: Consultancy; Epizyme: Consultancy; Daiichi Sankyo: Consultancy; Geron: Other: Clinical Trial Committees; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; BioCryst: Other: Clinical Trial Committees; BeyondSpring: Consultancy; Astex: Research Funding; Astellas: Consultancy; Aprea: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Agios: Consultancy; ADC Therapeutics: Research Funding; Acceleron: Consultancy, Research Funding; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding. Divino: IQVIA: Current Employment, Other: IQVIA received funding for this study from Epstein Health in connection with this study. DeKoven: IQVIA: Current Employment, Other: IQVIA received funding for this study from Epstein Health in connection with this study. Shah: IQVIA: Current Employment, Other: IQVIA received funding for this study from Epstein Health in connection with this study. Wang: IQVIA: Current Employment, Other: IQVIA received funding for this study from Epstein Health in connection with this study. Bey: Taiho Oncology: Current Employment. Salimi: Taiho Oncology: Current Employment. Epstein: Epstein Health: Current Employment; Fate Therapeutics: Other: Holds leadership position; Veracyte: Other: Holds leadership position; Illumina: Other: Holds leadership position; Merck: Consultancy; Radius Health: Consultancy; Halozyme: Consultancy; Intra-Cellular Therapies: Consultancy; Taiho Oncology: Consultancy; Otsuka: Consultancy.