This issue of Blood features valuable new data from Casulo et al1 focusing on an important problem in follicular lymphoma (FL). FL is the most common form of indolent non-Hodgkin lymphoma and the second most common type overall. After decades of minimal progress with chemotherapy-based treatments, availability of the anti-CD20 antibody rituximab (R) in 1997 led to significant improvements in overall survival.2 Initial management strategies for patients with advanced-stage disease now include observation for patients with asymptomatic, low tumor burden disease, whereas those requiring therapy may receive rituximab alone. Most patients receive rituximab (or the anti-CD20 obinutuzumab) in combination with chemotherapy such as bendamustine, cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP), or cyclophosphamide/vincristine/prednisone, which are sometimes followed by anti-CD20 antibody maintenance. Patients typically respond well to initial therapy, but relapse is expected within several years. Prognostic tools have been developed to predict outcomes, including the clinical Follicular Lymphoma International Prognostic Index (FLIPI)3 and a clinicogenetic risk model (m7-FLIPI).4 However, they are of limited value in choice of treatment, which is primarily driven by physician judgment and patient preferences rather than data demonstrating that one regimen is better than another for specific situations.
“A clever person solves a problem. A wise person avoids it.”
—attributed to Albert Einstein
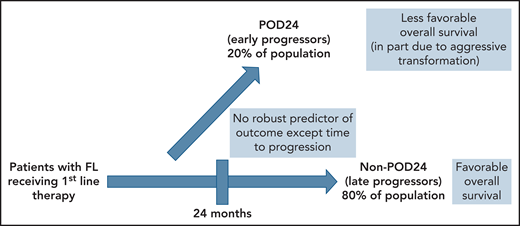
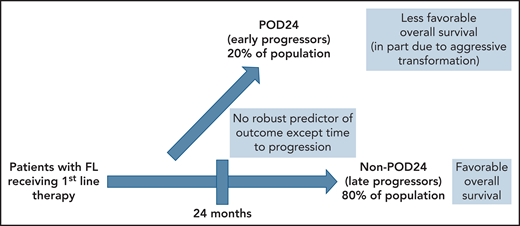
In 2015, previous work by Casulo et al5 from the National LymphoCare Study changed the way that many clinicians and researchers consider patients with recurrent FL. In more than 500 patients with FL initially treated with R-CHOP, about 20% had progression of disease within 2 years (the POD24 group). Five-year overall survival of these early progressors was 50%, as opposed to the non-POD24 group where it was 90%. Maurer et al6 have gone on to suggest that the latter group has an overall survival comparable to a similar population without lymphoma. The POD24 group, however, appears to represent a subgroup of patients that is in particular need of better approaches. The difficulty lies with the fact that someone with POD24 can only be identified after the patient has selected and received an initial regimen and the duration of the effect is subsequently observed (see figure).
Patients with FL fall into 2 major groups with respect to outcome. The subset of patients (approximately 80%) who progress at least 24 months after initial therapy (non-POD24) have a favorable outcome and near-normal life expectancy. The 20% of patients who progress within 24 months (POD24) have an unfavorable prognosis, in part because of the presence of histologic transformation at relapse. Presently, there is no reliable way to identify these 2 groups at diagnosis or target specific therapies to one group or the other.
Patients with FL fall into 2 major groups with respect to outcome. The subset of patients (approximately 80%) who progress at least 24 months after initial therapy (non-POD24) have a favorable outcome and near-normal life expectancy. The 20% of patients who progress within 24 months (POD24) have an unfavorable prognosis, in part because of the presence of histologic transformation at relapse. Presently, there is no reliable way to identify these 2 groups at diagnosis or target specific therapies to one group or the other.
The current report analyzed more than 5000 patients with FL receiving initial treatment in 13 clinical trials of the Follicular Lymphoma Analysis of Surrogate Hypothesis dataset, including studies conducted by the US National Cancer Institute National Clinical Trials Network (NCI NCTN), various academic institutions and international lymphoma study groups, and pharmaceutical companies.1 This work confirms the presence of POD24 as an important indicator of subsequent survival. Not surprisingly, those who had received rituximab alone may have had more favorable features (eg, less tumor burden), and therefore, early progression in this group after such less aggressive treatment was not as ominous. In a multivariable model, male sex, impaired performance status, elevated β-2-microglobulin, and high follicular FLIPI risk score were associated with POD24 and worse survival. These data reiterate that understanding of the FL POD24 population should be a major focus of our efforts as we try to improve overall survival through the rational application of new therapies.
Since the original observation of Casulo et al,5 much of our work has been directed toward being clever in trying to solve the POD24 problem. We now know that as many as three-fourths of patients with FL experiencing POD24 do so in the context of transformation to an aggressive histology at progression, likely an important negative influence on outcomes and survival.7 For this reason, biopsy at relapse of patients with FL with POD24 should be routinely considered. Multiple analyses of newer (and older) therapeutic approaches in patients with relapsed FL, including approved regimens such as lenalidomide/rituximab, phosphoinositide 3-kinase inhibitors, autologous stem cell transplantation, and CAR T cells, have demonstrated activity in patients with relapsed FL who had experienced POD24 with prior therapy.8,9 However, these FL cohorts are likely enriched for a nontransformed POD24 group that has more favorable outcomes by exclusion of the transformed POD24 group. For these reasons, it is uncertain how encouraging it is when a specific regimen is active in the POD24 population. Those of us that are clinical trialists in this area may not be as clever as we would like.
A wise approach might be to focus on trying to avoid the POD24 problem altogether through better identification of these patients at diagnosis and treating them with regimens that can avoid POD24 while remembering that POD24 may be a correlate with rather than a surrogate for survival.10 One approach is reflected in an ongoing NCI NCTN study led by the Southwest Oncology Group (S1608), which is not only comparing different treatment options for patients with FL after POD24, but is also working to develop tools to potentially identify POD24 before it happens. This trial is performing mutational analysis of tumor samples and validating the m7-FLIPI and assessing of circulating tumor DNA to potentially identify patients with POD24 before their disease progresses and even at initial diagnosis. Although we now have learned that patients with FL who are male and have high-risk FLIPI, impaired performance status, and/or elevated β-2 microglobulin are likely to do less well, we unfortunately do not know that our choice between rituximab, chemoimmunotherapies of various types or other options as initial treatment, consolidation, or maintenance leads to a change in their ultimate outcome. Additionally, for the 80% of patients who are without POD24 and less likely to die of their disease, quality of life (QOL) considerations might be the most important issue. Unfortunately, data on QOL to guide our choices are rudimentary at best. Being able to reliably identify newly diagnosed patients with FL that fall into different prognostic subgroups before they are treated is essential. Using this information to develop treatments that can either overcome a less favorable outlook or maximize QOL for those who have a normal life expectancy should be a high priority to ultimately benefit patients with FL. Our research and clinical efforts need to follow the advice of Theodore Roosevelt when he said “Nine-tenths of wisdom is being wise in time.”
Conflict-of-interest disclosure: J.P.L. has consulted for Sutro, Miltenyi, AstraZeneca, Epizyme, BMS/Celgene, Regeneron, Bayer, Gilead/Kite, Karyopharm, GenMab, Genentech/Roche, Abbvie, Incyte, Janssen, and Eisai.