Key Points
In patients with CLL/SLL who failed to respond to 2 BNT162b2 doses, close to a quarter responded to the third dose of vaccine.
Antibody-mediated responses were lower during active treatment and after exposure to anti-CD20 therapy.
Abstract
Patients with chronic lymphocytic leukemia (CLL) have an impaired antibody response to coronavirus disease 2019 (COVID-19) vaccination. Here, we evaluated the antibody response to a third BNT162b2 mRNA vaccine in patients with CLL/small lymphocytic lymphoma (SLL) who failed to achieve a humoral response after standard 2-dose vaccination regimen. Anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were measured 3 weeks after administration of the third dose. In 172 patients with CLL, the antibody response rate was 23.8%. Response rate among actively treated patients (12.0%; n = 12/100) was lower compared with treatment-naïve patients (40.0%; n = 16/40; OR = 4.9, 95% CI 1.9-12.9; P < .001) and patients off-therapy (40.6%; n = 13/32; OR = 5.0, 95% CI 1.8-14.1; P < .001), (P < .001). In patients actively treated with Bruton’s tyrosine kinase (BTK) inhibitors or venetoclax ± anti-CD20 antibody, response rates were extremely low (15.3%, n = 9/59, and 7.7%, n = 3/39, respectively). Only 1 of the 28 patients (3.6%) treated with anti-CD20 antibodies <12 months prior to vaccination responded. In a multivariate analysis, the independent variables that were associated with response included lack of active therapy (OR = 5.6, 95% CI 2.3-13.8; P < .001) and serum immunoglobulin A levels ≥80 mg/dL (OR = 5.8, 95% CI 2.1-15.9; P < .001). In patients with CLL/SLL who failed to achieve a humoral response after standard 2-dose BNT162b2 mRNA vaccination regimen, close to a quarter responded to the third dose of vaccine. The antibody response rates were lower during active treatment and in patients with a recent exposure (<12 months prior to vaccination) to anti-CD20 therapy. This trial was registered at www.clinicaltrials.gov as #NCT04862806.
Introduction
The coronavirus disease 2019 (COVID-19) is an ongoing global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clinical manifestations range from asymptomatic infection to life-threatening disease.1,2 Severe illness is more likely to occur in elderly patients and persons who have significant underlying medical conditions.1,2 Since 2021, variants of the virus have emerged and become dominant in many countries, with the delta variant being currently one of the most virulent.3
Patients with chronic lymphocytic leukemia (CLL) have an increased risk of severe COVID-19 and subsequent mortality.4,5 Recent reports have suggested that mortality from COVID-19 in patients with CLL has decreased over time, possibility due to better patient management.6 Seroconversion after the acute phase of SARS-CoV-2 infection is seen in 60% to 82% of patients with CLL.6-8 Patients with CLL may develop persistent COVID-19 infection as a result of their inability to effectively eliminate the virus. In such cases, prolonged shedding of infectious SARS-CoV-2 virus and additional in-host genomic evolution may eventually lead to development of new virus variants.6 Furthermore, the immune response to COVID-19 vaccination is reduced in patients with CLL/small lymphocytic lymphoma (SLL)9-11 and depends on continuing disease activity and treatment. It is particularly low during therapy at the time of vaccination.9,11
Administration of a third dose of mRNA-1273 COVID-19 vaccine to organ-transplant recipients 2 months after the second dose has been shown to be safe and increase antibody titers compared with placebo. Moreover, 44% of solid-organ transplant recipients who had been seronegative after 2 doses of BNT162b2 became seropositive after a third vaccine dose.12 In the light of these findings, we decided to evaluate the serologic response to a third BNT162b2 mRNA COVID-19 vaccine in patients with CLL/SLL who failed to achieve a humoral response after the standard 2-dose vaccination regimen.
Methods
This prospective study, conducted in the framework of the Israeli CLL study group, investigated the efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in seronegative patients with CLL/SLL who were followed at 7 medical centers in Israel. The study was approved by the institutional review board of each participating center and is registered at clinicaltrials.gov (#NCT04862806). All subjects provided informed consent and were vaccinated through a national Israeli vaccination program of administering a third vaccine dose to immunocompromised subjects. Eligibility criteria for the study included diagnosis of CLL/SLL according to the International Workshop on Chronic Lymphocytic Leukemia criteria,13 age 18 years or older, no known history of SARS-CoV-2 infection, and failure to obtain a serologic response 2 to 3 weeks after the second BNT162b2 vaccine dose.
Serum from the peripheral blood of patients with CLL/SLL was collected before and 3 weeks after administration of the third vaccine. The primary endpoint was to determine the proportion of subjects acquiring anti–SARS-CoV-2S antibodies. Serum samples were analyzed using the Architect AdviseDx SARS-CoV-2 IgG II (Abbot, Lake Forest, IL), which detects immunoglobulin G (IgG) antibodies to the receptor-binding domain (RBD) of the S1 subunit of the spike (S) protein, with a positive cutoff of >50 AU/mL.
A surrogate viral assay was used to test antiviral humoral response based on a highly infectious recombinant vesicular stomatitis virus bearing the SARS-CoV-2 spike glycoprotein S. The neutralizing activities were assessed in a high-throughput fluorescent reporter assay as previously published.14
All subjects were questioned about local or systemic adverse events that had occurred within 7 days after each vaccine dose. In patients who developed COVID-19 after vaccination, disease severity was defined according to the criteria of the World Health Organization.15 Relevant data were also extracted from the medical records and included demographics, complete blood count, Binet stage, serum immunoglobulin levels, mutational status of the immunoglobulin heavy chain variable (IGHV) gene (using a cutoff of 98% identity to the germ-line sequence), and analyses of genomic aberrations by fluorescent in situ hybridization (FISH),16 categorized according to the hierarchical model reported by Döhner et al.17
Statistics
IBM SPSS version 27 was used to apply the following information: descriptive statistics, including median interquartile range (IQR) range and mean. Kolmogorov-Smirnov test and Q-Q plot were used for evaluating normal distribution of quantitative data. Mann-Whitney U test and Kruskal-Wallis H test were used to compare continuous variables. Pearson's χ2 test or Fisher's exact test were applied for analyzing association between 2 categorical variables. Variables that were associated with a serologic response at a significant level of P < .1 in the univariate analysis were then included in the multivariate analysis performed by binary logistic regression. Log10 transformation was applied for normally distributed approximation of neutralizing antibody titers. Pearson's correlation coefficient between normally distributed quantitative variables was calculated. WINPEPI version 11.65 was used to calculate odds ratio (OR) and its 95% confidence interval (CI) and the mid-p exact CI when ≥1 cells had a limited number of subjects. Statistical significance was determined as P < .05, and all statistical tests were 2-sided.
Results
Patient characteristics
From July 2020 through August 2021, a total of 172 patients with CLL/SLL were included in the study. (Patient baseline demographic and disease characteristics are summarized in Table 1.) All patients failed to produce anti–SARS-CoV-2S antibodies after the standard 2-dose BNT162b2 mRNA vaccination regimen and were seronegative before the third vaccine dose.
The median age was 72.1 years (IQR, 68.1-77.7), and 121 patients (69.9%) were men. Forty patients (23.3%) were treatment-naïve, 100 (58.1%) were on active therapy, and 32 (18.6%) were previously treated (off-therapy). Among the off-therapy patients, 24 (75%) were in remission (complete remission: n = 18; partial remission: n = 6) and 8 (25%) were in relapse. The median time from CLL diagnosis to the third vaccination was 63.5 months (IQR, 61-162), the median time from second to the third vaccine was 179 days (IQR, 175-187), and the median time from the third vaccine dose to serology testing was 21 days (IQR, 21-22). Furthermore, in patients on-therapy, the median time from initial treatment to third vaccination was 17.7 months (IQR, 8.3-36.4).
Serologic response
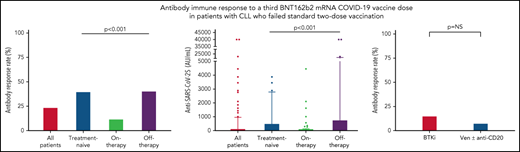
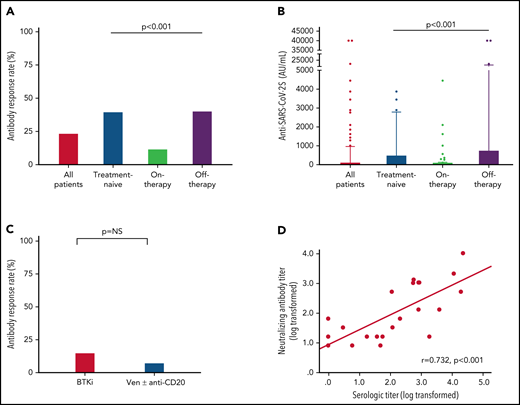
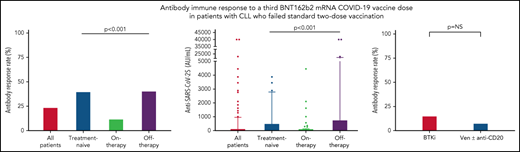
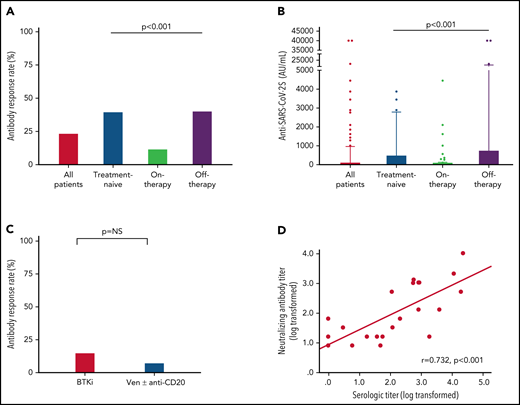
Antibody response to the third BNT162b2 mRNA dose was seen in 41 of 172 (23.8%; Table 2) patients with a median antibody level of 2 AU/mL (IQR, 0-40.8; supplemental Table 1; Figure 1A-B). In a univariate analysis, the variables that were significantly associated with response were lack of active therapy (including previously untreated patients and those off-therapy; OR = 5.0, 95% CI 2.2-11.5; P < .001), ≥12 months from the last anti-CD20 therapy to the third vaccination (22.7% vs 3.6%; P = .033), and serum IgG and IgA levels of ≥550 and ≥80 mg/dL, respectively (OR = 2.58, 95% CI 1.02-7.29; P = .047; and OR = 5.4, 95% CI 2.2-13.9; P < .001; respectively) (Table 2). In patients aged ≤65 years, antibody titers were higher (median = 12 AU/mL; IQR, 0-40.8) compared with older patients (>65 years) (median 1 AU/mL, IQR, 0-422; P = .034; supplemental Table 1); however, there was no statistically significant difference in response rates (36.4% [n = 12/33] vs 20.9% [n = 29/139]; OR = 2.2, 95% CI 0.9-5.3; P = .06; Table 2). Treatment-naïve patients as well as those off-therapy had higher response rates (40.0% [n = 16/40] and 40.6% [n = 13/32], respectively; Table 2; Figure 1A) and higher antibody levels (median = 8 [IQR, 0.3-509.8], median = 6 [IQR, 0.0-764.5], respectively; Figure 1B; supplemental Table 1) compared with actively treated patients (12.0%; n = 12/100; OR = 4.9, 95% CI 1.9-12.9; P < .001; and OR = 5.0, 95% CI 1.8-14.1; P < .001, respectively; Table 2; and median = 0.0, IQR, 0-10.3; Figure 1A-B; supplemental Table 1; P < .001). Among patients off-therapy, the serologic response rates among patients in complete remission, partial remission, and relapse were 38.9%, 50%, and 37.5%, respectively (P = .894). The median time from last treatment to the third vaccine dose in these patients was 11.5 months (IQR 7.3-51.6) in the responders compared with 10.2 months (IQR 4.0-20.9) in the nonresponders (P = .465).
Antibody response rate and titers after a third vaccine dose in patients with CLL who failed to respond after the standard 2-dose BNT162b2 mRNA vaccination regimen. (A-B) Antibody response rate (%) and anti–SARS-CoV-2 antibody levels in patients with CLL shown for the entire cohort and according to the disease status: all CLL patients (n = 172); treatment naïve (n = 40); on-therapy (n = 100); and off-therapy (n = 32). (C) Response rate in patients with CLL treated with Bruton's tyrosine kinase inhibitor (BTKi; n = 59) and venetoclax (Ven) ± anti-CD20 antibody (n = 39). (D) Correlation between serological titers and neutralizing antibody levels following log transformation (n = 24), (Pearson's correlation coefficient r = 0.732; P < .001; r2 = 0.536). In an additional 30 patients, the anti–SARS-CoV-2 and neutralizing antibodies levels were negative and therefore were invalid for analysis.
Antibody response rate and titers after a third vaccine dose in patients with CLL who failed to respond after the standard 2-dose BNT162b2 mRNA vaccination regimen. (A-B) Antibody response rate (%) and anti–SARS-CoV-2 antibody levels in patients with CLL shown for the entire cohort and according to the disease status: all CLL patients (n = 172); treatment naïve (n = 40); on-therapy (n = 100); and off-therapy (n = 32). (C) Response rate in patients with CLL treated with Bruton's tyrosine kinase inhibitor (BTKi; n = 59) and venetoclax (Ven) ± anti-CD20 antibody (n = 39). (D) Correlation between serological titers and neutralizing antibody levels following log transformation (n = 24), (Pearson's correlation coefficient r = 0.732; P < .001; r2 = 0.536). In an additional 30 patients, the anti–SARS-CoV-2 and neutralizing antibodies levels were negative and therefore were invalid for analysis.
Among the 100 patients on treatment at the time of vaccination, 99 (99%) were treated with novel agents, including Bruton's tyrosine kinase (BTK) inhibitors (ibrutinib or acalabrutinib; n = 59) and venetoclax ± anti-CD20 antibody (rituximab or obinutuzumab; n = 39), and 1 patient was treated with idelalisib. Antibody response rate in patients receiving BTK inhibitors was 15.3% (n = 9/59) compared with 7.7% (n = 3/39) in patients treated with venetoclax ± anti-CD20 antibodies (OR = 2.2, 95% CI 0.6-10.4; P = .353; Figure 1C; Table 2). Five patients were treated with venetoclax alone, of whom 3 (60.0%) responded. Among patients on active treatment, the median time since start of therapy to the third vaccine dose was 16.4 months (IQR 7.6-34.5) in responders compared with 25.4 months (IQR 8.9-46.3) in the nonresponders (P = .376).
A total of 94 patients with CLL had been previously exposed to anti-CD20 therapy: 28 patients within the last 12 months prior to vaccination (21 patients <6 months and 7 patients between 6 and 12 months; median = 3.9 months, IQR, 1.2-6.6) and 66 patients ≥12 months before vaccination (median = 49.2 months, IQR, 19.1-70.7). Most patients (n = 24, 85.7%) exposed to anti-CD20 antibodies <12 months prior to vaccination received it in combination with venetoclax. Only 1 of the 28 patients (3.6%) treated with anti-CD20 antibodies within the last 12 months has responded vs 22.7% (n = 15 of 68) of those exposed to anti-CD20 therapy ≥12 months prior to vaccination (P = .033; Table 1). In patients who received anti-CD20 antibody ≥12 months, there was no statistically significant difference in median of time since last anti-CD20 treatment between responsive (64.5 months, IQR, 18.3-83.6) and nonresponsive patients (44.3 months, IQR, 20.5-66.5) (P = .288). However, after controlling for other active treatments, each month that elapsed from the end of administration of the anti-CD20 therapy increased the odds for response by 1.03 times (OR = 1.03, 95% CI 1.01-1.05; P = .020). In a multivariate analysis (Table 3), the independent variables associated with response included lack of active therapy (OR = 5.6, 95% CI 2.3-13.8; P < .001) and serum IgA levels ≥80 mg/dL (OR = 5.8, 95% CI 2.1-15.9; P < .001).
Samples taken from 54 patients were also evaluated for production of neutralizing antibodies. As shown in Figure 1D, the anti–SARS-CoV-2 RBD antibody levels linearly correlated with neutralizing antibodies titers (log transformed, r = 0.732 and P < .001).
Within a median follow-up period of 60 days (IQR, 60-75) since the third vaccine dose, 4 patients developed COVID-19 infection, including 2 with severe disease and 2 with mild disease (supplemental Table 2). Two patients were actively treated with ibrutinib; 1 was treatment-naïve and 1 had been previously treated with obinutuzumab monotherapy. Overall, 1 patient died because of COVID-19 whereas all the others recovered. Among these patients, 3 were seronegative after a third vaccine dose and another was seropositive with a low antibody titer (138 AU/mL).
Adverse events
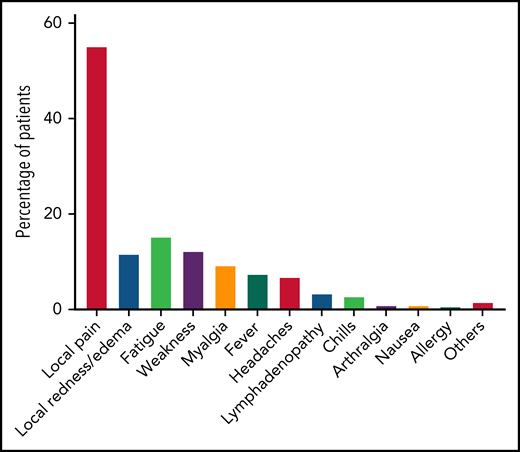
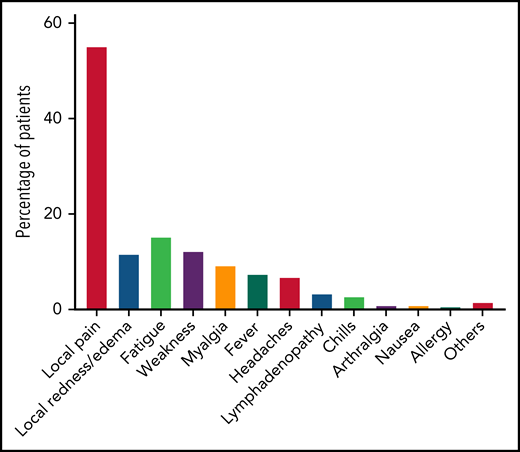
A total of 168 patients were available for evaluation of adverse events after the third dose of the vaccination (Figure 2; supplemental Table 3). Local adverse events included pain at the injection site, which was reported in 93 patients (55.4%), and local erythema or swelling, which was reported in 20 patients (11.9%). Overall, 45 patients (26.8%) reported at least 1 systemic adverse event, all of which were mild. The most frequently reported systemic reactions included fatigue (n = 26, 15.5%), weakness (n = 21, 12.5%), myalgia (n = 16, 9.5%), and fever (n = 13, 7.7%). No statistically significant association was found between local or systemic reaction and a positive serological response to the third vaccination.
Adverse events reported after the third BNT162b2 vaccine dose in patients with CLL (n = 168).
Adverse events reported after the third BNT162b2 vaccine dose in patients with CLL (n = 168).
Discussion
This study evaluated the serologic response to a third BNT162b2 mRNA vaccine dose in patients with CLL/SLL who had failed to respond to the standard 2-dose vaccination program. The overall response rate was 23.8%, whereas in univariate analysis, lack of active treatment ≥12 months from the last anti-CD20 therapy and higher serum IgG and IgA levels were associated with a better response rate. In multivariate analysis, lack of active therapy and serum IgA levels ≥80 mg/dL were the only independent variables associated with serologic response. In our cohort, the response rate was lower than the 44% reported for organ transplant recipients, who had been seronegative after 2 doses of BNT162b2 and responded to the third vaccine dose.12 This difference can be attributed to the complex inherent immune deficiency in patients with CLL, the timing of the third vaccine dose (60 days in the solid-organ transplant recipients versus 180 days in the patients with CLL), and the different therapy modalities used. Whereas CLL treatment essentially focuses on targeting B cells, in organ transplant recipients, therapy is directed primarily against T cells.18
In treatment-naïve and previously treated patients, the response rate after vaccination approached 40% compared with only 12% in those currently on active therapy. Most of the latter were treated with BTK inhibitors or venetoclax plus anti-CD20 antibody. Treatment with ibrutinib has been reported to result in partial reconstitution of humoral immunity and normal B-cell subpopulations in patients with CLL, but at the same time, it also decreased serum IgG levels starting at 6 months of therapy and becoming more profound by 24 months. The poorer response to the vaccine given after exposure to rituximab or obinutuzumab within the last 12 months prior to vaccination and the improved incremental response after 12 months are consistent with the kinetics of rapid peripheral blood B-cell depletion after anti-CD20 therapy, followed by a recovery starting between 6 and 9 months after treatment with a return to normal counts at 12 months,19 even after taking into consideration the relatively low number of patients treated with venetoclax monotherapy. The response rate still appeared to be higher compared with that seen after BTK inhibitors or with venetoclax plus anti-CD20 antibody. This also seemed to be the case after administration of the second dose of vaccine.20,21
In cases of CLL, hypogammaglobinemia is consistently associated with lower humoral responses to COVID-19 vaccination,9,20,22 even after the third vaccination. Low immunoglobulin levels are commonly encountered in patients with CLL and decrease even more with duration and progression of disease.23,24 Hypogammaglobinemia is a major risk factor for infections in patients with CLL,24 and it is known that immunization with other vaccines is more efficient if immunoglobulin levels are better preserved.25
Overall, the response after the third vaccine had a pattern similar to that observed after the first 2 doses given in patients with CLL, which correlated with the degree of immunosuppression accompanying the disease and therapy. Whereas in our study patients received a homologous mRNA vaccine regimen, a recent report suggested that heterologous prime-boost vaccination may indeed facilitate a stronger response.26 Given the low response rate evident in our cohort, the latter approach in patients with CLL deserves further investigation.
In addition, higher anti–SARS-CoV-2 S-RBD IgG titers were associated with younger age (≤65 years), lack of active treatment, and higher serum IgG and IgA levels. Similarly, we and others have already reported a better response rate and higher antibody titers after 2 BNT162b2 vaccine doses in younger patients with CLL.9,27 The anti–SARS-CoV-2 S-RBD IgG titers also linearly correlated with neutralizing antibodies titers, similar to our findings recorded after the second BNT162b2 dose in patients with CLL.20 Recently, anti–SARS-CoV-2 levels have been shown to be clinically meaningful in terms of protection against COVID-19.28 Among fully vaccinated healthcare workers, the occurrence of breakthrough infections with SARS-CoV-2 correlated with the levels of neutralizing antibodies and SARS-CoV-2S antibodies, measured both within the first month after the second vaccine dose and peri-infection.28
CD8 T-cell immunity has been shown to be a critical parameter for survival in patients with hematologic malignancy with COVID-19,29 and vaccination with 2 doses of BNT162b2 has been reported to induce cellular response in only approximately half of patients with hematologic malignancies.27 The T-cell response generally correlated with the anti-S IgG levels, but some patients who had no humoral response also achieved T-cell responses.27 Nevertheless, the effects of a third COVID-19 vaccine dose on cellular immunity still need to be studied in patients with CLL to better understand its protective role in patients who failed to achieve a humoral response.
In immunocompetent individuals, immunity after BNT162b2 vaccination has been reported to wane after 6 months.30 Neutralization levels are highly predictive of immune protection,31 and a BNT162b2 booster dose can increase the antibody neutralization level by an average of 5 to 7 times, compared with that obtained after a second dose.32 In subjects aged ≥60 years, a booster dose of the BNT162b2 vaccine has been shown to substantially lower the number of confirmed cases of COVID-19 and decrease severity of the disease.33 The potential risk for severe COVID-19 disease and its complications in patients with CLL outweigh the lack of vaccine response. Although our study focused primarily on the humoral reactivity in patients who failed to respond to the initial vaccination, we suggest that a booster be considered for all patients with CLL who had been vaccinated with mRNA vaccines, including those receiving the booster while still on active anti-CLL therapy. On a case-by-case basis, it may be appropriate to delay the start of therapy to allow for COVID-19 vaccination. Patients who have not responded to the booster and have been treated with fixed-duration treatment (eg, including with anti-CD20 antibody) may still respond to an additional booster dose after a period of time that would allow for immune reconstitution; however, the vaccine type to be used (eg, heterologous prime-boost vaccination) and optimal timing still need to be studied. Until now, there has been no recommendation for routine serology testing in patients with CLL after COVID-19 vaccination. However, knowing the degree of response obtained would probably help with improved risk stratification and future management. In this respect, regardless of the immune response to vaccination, it is important that patients with CLL continue to take all necessary precautions.
In summary, it is noteworthy that almost a quarter of the patients with CLL/SLL who failed to achieve a humoral response after standard 2-dose BNT162b2 mRNA vaccination regimen responded to the third dose of vaccine. The antibody-mediated response continued to be markedly impaired during active treatment and after recent exposure (<12 months prior to vaccination) to anti-CD20 therapy. These findings are of practical importance because they show that a third dose can still achieve seroconversion even in the more immunosuppressed subgroup of patients with CLL.
Acknowledgments
The authors thank the clinical study coordinators and nurses of the hematology department at the Tel-Aviv Sourasky, Bnai Zion, Rabin, and Chaim Sheba medical centers. The authors also thank Gert Zimmer, University of Bern, Bern, Switzerland, for providing the high-throughput fluorescent reporter assay.
This work was supported by a grant by Janssen Pharmaceuticals (EV00261620).
Authorship
Contribution: Y.H. initiated the trial, designed the study, collected and analyzed data, and wrote the paper; O. Benjamini and T.T. initiated the trial, designed the study, and collected the data; S.L. and T.Z.-B. analyzed the data; O. Bairey, G.R., A.B., G.I., N.D., and L.S. collected the data; and A.P. provided critical reading of the manuscript.
Conflict-of-interest disclosure: Y.H. reported honoraria from AbbVie, Janssen Pharmaceuticals, AstraZeneca, Medison, and Roche outside the submitted work. T.T. reported honoraria from AbbVie, Janssen Pharmaceuticals, AstraZeneca, Novartis, Pfizer, and Roche outside the submitted work. O. Benjamini reported honoraria from AbbVie, Janssen Pharmaceuticals, and AstraZeneca outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Yair Herishanu, Department of Hematology, Tel Aviv Sourasky Medical Center, 6 Weizmann St, Tel Aviv 64239, Israel; e-mail: yairh@tasmc.health.gov.il.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
REFERENCES
Author notes
T.T. and O. Benjamini contributed equally to this study.