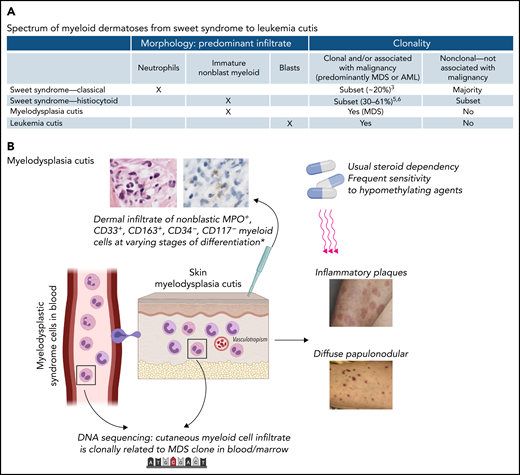
In this issue of Blood, Delaleu et al1 provide compelling evidence for formal recognition of an entity termed “myelodysplasia cutis” (MDS cutis) with histologic features of histiocytoid Sweet syndrome in the skin and a clonal relationship to myelodysplastic cells in the bone marrow.
In 1964, Robert Sweet first reported 8 patients with an acute febrile neutrophilic dermatosis, later termed Sweet syndrome.2 Classical Sweet syndrome is histologically characterized by an inflammatory dermal infiltrate composed primarily of mature neutrophils. Sweet syndrome is associated with many etiologies, including infection, inflammatory disease, pregnancy, drug-related (commonly granulocyte colony-stimulating factor), and in 20% of cases is associated with malignancy (see figure panel A). The most common associated malignancies are myeloid3 and include myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML).
(A) Spectrum of myeloid dermatoses, including classical Sweet syndrome, histiocytoid Sweet syndrome, the proposed entity of MDS cutis, and leukemia cutis. The morphology of the predominant skin infiltrate in each entity is indicated. The clonality or association of each entity with malignancy is also shown. The most common malignancies are myeloid malignancies. (B) MDS cutis. MDS cells originating in the bone marrow circulate in the peripheral blood, entering the skin and forming a dermal infiltrate with histologic features of so-called histiocytoid Sweet syndrome. On skin biopsy, the infiltrate is MPO+, CD33+, CD163+, MPO+, CD34−, and CD117−. This immunophenotype represents nonblast myeloid precursors at varying stages of differentiation and myelomonocytic cells. DNA sequencing of bone marrow and skin identify the presence of the same mutations, providing evidence that the cutaneous myeloid cells are clonally related to the MDS cells in the marrow. MDS cutis may present as inflammatory plaques or diffuse papulonodular lesions. Lesions may respond to steroids with dependency, although there is frequent response to hypomethylating agents in this study.
(A) Spectrum of myeloid dermatoses, including classical Sweet syndrome, histiocytoid Sweet syndrome, the proposed entity of MDS cutis, and leukemia cutis. The morphology of the predominant skin infiltrate in each entity is indicated. The clonality or association of each entity with malignancy is also shown. The most common malignancies are myeloid malignancies. (B) MDS cutis. MDS cells originating in the bone marrow circulate in the peripheral blood, entering the skin and forming a dermal infiltrate with histologic features of so-called histiocytoid Sweet syndrome. On skin biopsy, the infiltrate is MPO+, CD33+, CD163+, MPO+, CD34−, and CD117−. This immunophenotype represents nonblast myeloid precursors at varying stages of differentiation and myelomonocytic cells. DNA sequencing of bone marrow and skin identify the presence of the same mutations, providing evidence that the cutaneous myeloid cells are clonally related to the MDS cells in the marrow. MDS cutis may present as inflammatory plaques or diffuse papulonodular lesions. Lesions may respond to steroids with dependency, although there is frequent response to hypomethylating agents in this study.
A histologic variant of Sweet syndrome called “histiocytoid” Sweet syndrome is composed of a mononuclear dermal infiltrate that morphologically resembles histiocytes because of elongated, twisted, or kidney-shaped vesicular nuclei of infiltrating cells.4 However, the infiltrate in histiocytoid Sweet syndrome was demonstrated to contain cells expressing myeloperoxidase (MPO), CD163, CD33, CD68, and lysozyme, which were negative for CD34 and CD117.4 This immunophenotype was consistent with nonblast myeloid precursors and myelomonocytic cells. Depending on the study, the association of histiocytoid Sweet syndrome with malignancy ranges from 30% to 61%.5,6 The most common associated neoplasia is MDS.5
In patients with myeloid neoplasia and Sweet syndrome, fluorescence in situ hybridization can detect the same cytogenetic aberrancies in bone marrow and skin lesions, demonstrating a clonal link between the neoplastic cells in the marrow and the infiltrate in the skin.7 Interestingly, some cases with AML in the marrow have been clonally linked to a mature neutrophilic infiltrate in the skin, suggesting that the skin infiltrate in some cases may represent differentiated cells from the blast clone in the marrow.7
The current study expands upon prior studies by utilizing next-generation sequencing panels and Sanger sequencing to evaluate skin biopsies of patients with histiocytoid Sweet syndrome and MDS. The authors sought to determine whether the MDS-related myeloid mutations in the bone marrow or blood were also present in the skin lesions. Seven patients were evaluated. The authors were able to detect the same mutations that were present in the marrow in the skin lesions with relatively high variant allele frequencies indicating that the mutations were from skin infiltration and not peripheral blood contamination. The myeloid genes in which the mutations were detected included ASXL1, CUX1, DNMT3A, RUNX1, SRSF2, STAG2, TET2, U2AF2, and ZRSR2. Notably, 1 patient was found to have a mutation in ubiquitin-activating enzyme 1 (UBA1) in both marrow and skin by Sanger sequencing. This patient had clinical symptoms of the recently described VEXAS (Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic) syndrome,8 which is a severe systemic autoinflammatory disease with predisposition to developing MDS, plasma cell neoplasia (monoclonal gammopathy of undetermined significance or myeloma), and venous thromoboembolism.9 In the original VEXAS cohort, 88% of patients had skin involvement and 32% was histologically diagnosed with Sweet syndrome.8 As in nearly all VEXAS cases reported to date, vacuoles were present in myeloid and erythroid precursors on evaluation of marrow aspirate smears. This case adds to the growing number of VEXAS cases reported in the literature.
The authors make a reasonable argument for the formal recognition of a diagnostic entity termed “myelodysplasia cutis” (see figure panel B) or “MDS cutis” for histiocytoid Sweet syndrome occurring in patients with MDS. This is particularly relevant given that the cells in the dermal infiltrate have been shown to be clonally related to MDS in the marrow and blood. The diagnosis of MDS cutis is more specific and removes these cases from the general classification of histiocytoid Sweet syndrome, which encompasses many other etiologies. The designation of MDS cutis may also have important implications for treatment. The frontline treatment of classical and histiocytoid Sweet syndrome is systemic corticosteroids. Based on the preliminary findings in the current study, MDS cutis may respond better to hypomethylating agents, which are directed at the underlying neoplastic myeloid clone, underscoring the relevance of appropriate diagnostic terminology.
On the spectrum of myeloid dermatoses, the most ominous form is leukemia cutis, characterized by a dermal infiltrate primarily composed of blasts. Survival is significantly greater for MDS cutis than leukemia cutis, and the diagnostic distinction between these 2 entities is critical.10 The blasts in leukemia cutis often express CD34, CD117, and/or CD56, markers, which are largely negative in Sweet syndrome and MDS cutis. In 1 study, MDS cutis preceded the diagnosis of MDS in the marrow in over half of the patients.10 In contrast, leukemia cutis occurred after AML was diagnosed in the marrow. The diagnosis of leukemia cutis in a patient with MDS has a poor prognosis and may herald the development of overt AML.
In contrast to older classifications based largely on morphologic features, in the last decade, disease classification of hematolymphoid neoplasia has moved toward a molecular, genetic, and clinically relevant classification. One could argue that nonblast myeloid dermatoses clonally related to MDS cells in the marrow could be designated MDS cutis regardless of whether the infiltrate appears neutrophilic (as in classical Sweet syndrome) or “histiocytoid.” “Histiocytoid” Sweet syndrome is a morphologic classification that is particularly confounding in patients with MDS given that the cells have not been demonstrated categorically to be histiocytes, but rather to represent immature nonblast myeloid precursors and myelomonocytic cells that are part of the MDS clone.
In summary, the spectrum of myeloid dermatoses includes (1) classical Sweet syndrome represented by an inflammatory dermal infiltration composed primarily of terminally differentiated neutrophils, (2) histiocytoid Sweet syndrome with morphologically immature nonblast myeloid cell infiltrate, and (3) leukemia cutis represented by a blast infiltrate in the skin. MDS cutis represents an emerging entity on this spectrum between histiocytoid Sweet syndrome and leukemia cutis, with a dermal infiltrate composed of nonblast myeloid cells clonally related to MDS cells in the marrow.
Conflict-of-interest disclosure: The author declares no competing financial interests.