In this issue of Blood, Romano et al discover that in murine bone marrow, both granulocyte and erythroid precursors develop around a single central macrophage, within a specialized niche termed erythromyeloblastic islands (EMBI).1 Although it is well established that mammalian erythropoiesis occurs in erythroblastic islands (EBI), comprising 5 to 30 maturing red blood cell precursors surrounding a macrophage,2 like an orchestra around a conductor, Romano et al identify that the “orchestra” constitutes granulocyte as well as erythroid precursors. Furthermore, they introduce a new concept in hematopoietic cell homeostasis: an inverse relationship between erythroid and granulocyte lineage cell numbers in EMBI that vary in response to microenvironmental cues. These findings provide insight into disorders such as anemia of inflammation/chronic disease.
The precise roles of EBI macrophages in erythropoiesis are not fully understood, but include cross-talk between erythroblasts and macrophages, critical for erythroblast survival, and macrophage engulfment of extruded erythroid nuclei in terminal erythropoiesis.2 EBI dysfunction is increasingly recognized as contributing to the pathogenesis of anemia in diseases such as thalassemia3 and myelodysplastic syndromes,4 yet the regulatory mechanisms controlling EBI and their role in nonerythroid lineage development are unknown. In part, this is because studying EBI is fraught with technical challenges2: first, the immunophenotype of EBI macrophages remains incompletely defined; second, macrophages are prone to fragmentation during manipulation; and third, purification of these cells may be hampered by their low frequency in bone marrow, tight adherence to other cell types and ingestion of erythroid cells resulting in “membrane switching,” making accurate identification and purification of macrophages by their cell surface antigen expression difficult.
Romano et al address these challenges using density centrifugation of bone marrow from wild-type C57BL/6J mice to enrich for cell clusters. They then identify EBI by multispectral imaging flow cytometry, a technique that allows cell enumeration by flow cytometry, combined with morphological assessment by imaging. EBI macrophages are further characterized by confocal microscopy, single-cell RNA-sequencing and cellular indexing of transcriptomes and epitopes-sequencing (CITE-seq).
The first observation is that ∼75% of “EBI” contain cells expressing CD11b (αMβ2 integrin). These are morphologically identifiable as granulocyte precursors, mainly myelocytes and metamyelocytes, and are interspersed with erythroid lineage cells expressing CD71 (the transferrin receptor) and spanning the erythroid hierarchy, from CD34+ erythroid progenitors to erythroblasts to occasional anuclear reticulocytes. EBI are therefore more accurately termed “EMBI,” comprising both erythroid and myeloid cells in close contact with a macrophage.
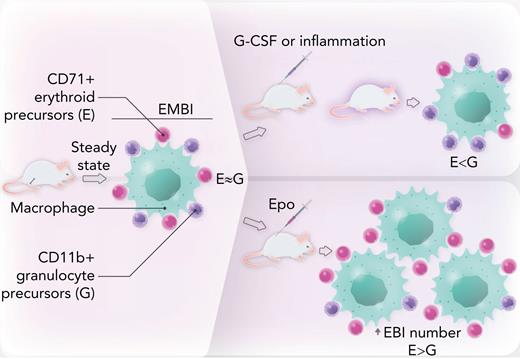
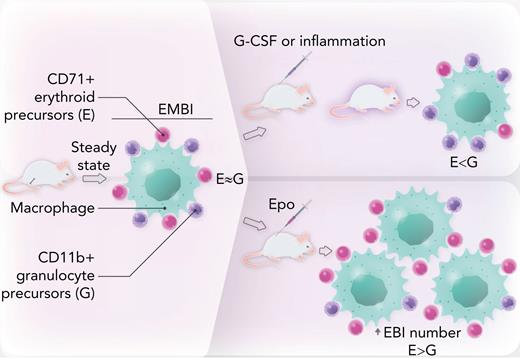
Next, the authors show that in steady state, the ratio of CD71+ erythroid to CD11b+ granulocytes precursors in EMBI is approximately 1.4:1; however, this ratio is modulated by intrinsic or extrinsic factors (see figure). Specifically, genetic knockdown of the myeloid transcription factor Gfi1, a model for congenital neutropenia, inhibits granulopoiesis as expected, and in turn increases the relative proportion of erythroid cells. In contrast, pharmacologic treatment of mice with granulocyte colony-stimulating factor stimulates granulopoiesis, with a corresponding dose-dependent reduction in erythropoiesis. Murine models of inflammation (interleukin-10 knockdown resulting in inflammatory bowel disease and cecal ligation peritonitis/sepsis) significantly decrease the CD71+:CD11b+ ratio, leading to anemia and leukocytosis.
EMBI in mouse bone marrow are composed of a central macrophage or “nurse” cell surrounded by a rosette of maturing granulocyte and erythroid precursors. At steady state, there are similar numbers of these 2 lineages. In mice treated with granulocyte colony-stimulating factor or in models of inflammatory disease, there is a shift toward granulocyte precursors, leading to anemia. Conversely, in mice treated with Epo, erythroid cells are increased relative to their granulocyte counterparts within EMBI. Epo also promotes an absolute increase in EMBI numbers. Professional illustration by Somersault18:24.
EMBI in mouse bone marrow are composed of a central macrophage or “nurse” cell surrounded by a rosette of maturing granulocyte and erythroid precursors. At steady state, there are similar numbers of these 2 lineages. In mice treated with granulocyte colony-stimulating factor or in models of inflammatory disease, there is a shift toward granulocyte precursors, leading to anemia. Conversely, in mice treated with Epo, erythroid cells are increased relative to their granulocyte counterparts within EMBI. Epo also promotes an absolute increase in EMBI numbers. Professional illustration by Somersault18:24.
Conversely, treatment of mice with erythropoietin (Epo) increases the erythroid to myeloid cell ratio in EMBI. Furthermore, Epo elevates the absolute number of EMBI by approximately fourfold. Because Epo is the major growth factor stimulated in response to anemia or hypoxia, these data confirm prior reports of a critical role for EBI in stress erythropoiesis5 and further show that Epo-induced erythropoiesis occurs at the expense of granulopoiesis.
Previous work has suggested that EBI macrophages express the Epo receptor (EpoR), as well as multiple receptors for erythroblast cell surface ligands.6 Romano et al confirm expression of previously described markers of EBI macrophages including F4/80 (detectable in mouse but not human EBI) and VCAM1, with heterogeneous expression of CD169.7 Surprisingly, given their Epo-responsiveness, fewer than 10% of macrophages express the EPOR transcript, measured at single-cell resolution. Further work is needed to reconcile this discrepancy, but 1 possible explanation is that in the EpoR-eGFPcre knockin mouse model used by Li and colleagues,6 macrophages were contaminated by ingested fluorescent erythroid nuclei.
Limitations of flow cytometry include the ability to examine only a finite number of proteins determined a priori and the lack of contemporaneous transcriptomic data in single cells. The authors therefore characterize EMBI by CITE-seq and show that Epo treatment significantly alters the surface protein phenotype, but not the transcriptome, of EBI macrophages. The authors ascribe this finding to macrophage heterogeneity in the EMBI; however, a caveat to this conclusion is the variability introduced by contaminating cell types because of the previously mentioned technical challenges associated with isolating and analyzing macrophages. Future work using techniques that increase both the yield and purity of isolated macrophages will more accurately define true macrophage heterogeneity within EMBI. The authors find that the major macrophage subsets, proinflammatory “M1” and anti-inflammatory “M2,” are equally prevalent in EMBI; however, macrophage polarization by cytokines and growth factors has previously been reported,8,9 and this area requires further clarification.
In summary, Romano et al provide evidence for a previously unknown homeostatic mechanism in normal murine bone marrow, whereby erythroid and granulocyte precursors, which stem from a common multipotent progenitor, are coregulated by microenvironmental factors in EMBI. Tantalizingly, they allude to preliminary data supporting the presence of EMBI in human bone marrow. Further studies will be needed to elucidate the role of EMBI in anemia resulting from the bone marrow inflammatory milieu that typifies chronic diseases10 or from the unchecked stimulation of granulopoiesis in myeloproliferative disorders. The observation that pathological expansion of 1 of the lineages within this niche may suppress the other means that interactions within EMBI could be a useful therapeutic target for these disorders and other cytopenias. Although it is known that specialized “stress erythroid progenitors” are induced during stress erythropoiesis,5 this work also postulates that high-Epo conditions may lead to both quantitative and qualitative changes in EMBI macrophages. What remains to be elucidated are the identity and protean roles of EMBI macrophages in physiological and pathological human erythropoiesis.
Conflict-of-interest disclosure: The author declares no competing financial interests.