In this issue of Blood,Holmes et al report that oral administration of galactooligosaccharides (GOS) as a prebiotic to laboratory mice significantly attenuated lethal graft-versus-host disease (GVHD) in a major histocompatibility complex–disparate model of allogeneic hematopoietic cell transplantation (allo-HCT).1
As food ingredients, GOS are complex carbohydrates with polymers of galactose and N-terminal glucose sugars that the host cannot digest in the upper gastrointestinal tract because of the enzymatic repertoire. In the colon, these sugars are fermented by microbes and provide a food source for energy and growth for members of the commensal microbiome such as Lactobacillus and Bifidobacterium spp.2 However, in the Holmes et al study, the beneficial effect of GOS on GVHD was observed only in mice treated with antibiotics such as imipenem-cilastatin, and these antibiotics are believed to disrupt the gut microbiome in these mice.
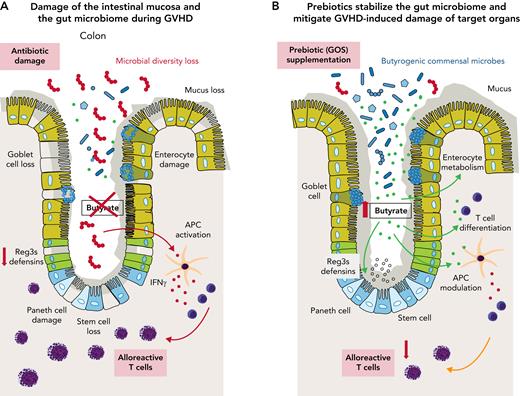
Several lines of evidence suggest that disruption of the gut microbiome induced by chemotherapy, dietary deficiencies, and/or antibiotics is associated with increased incidence of GVHD, a major cause of morbidity and mortality in patients who have received allo-HCT. The peritransplant use of broad-spectrum antibiotics such as imipenem-cilastatin or piperacillin-tazobactam has been associated with gut microbiome dysbiosis and increased GVHD-related mortality in patients.3 Current models of GVHD propose that broad-spectrum antibiotics deplete Clostridium spp. and other commensal bacteria in the gut that produce short-chain fatty acids (SCFAs; see figure panel A). Loss of butyrate and other SCFAs can disrupt enterocyte homeostasis and impair the differentiation or expansion of regulatory T cells in the intestinal mucosa. SCFAs also regulate the expression of antimicrobial peptides (eg, Reg3β) on gut mucosal surfaces as host defense mechanisms against pathogens and facultative pathogenic commensals.4 All these factors are critical for the development of GVHD and have an impact on its severity.5 Therefore, recent research has focused on modulating alloreactive T cells by using microbial metabolites6 or on restoring microbial homeostasis after allo-HCT by using microbiome interventions such as fecal microbiome transfers.7
The panels show one concept of GVHD prevention and/or therapy by prebiotic stabilization of the gut microbiome. (A) GVHD-associated disruption of gut mucosa homeostasis that is (partly) fueled by antibiotic-induced microbiome damage. (B) GOS prebiotics enhance the metabolic capacity of the microbiome to produce more butyrate and other SCFAs that can modulate alloreactivity and inflammatory processes in the allo-HCT recipient. APC, antigen-presenting cell; IFNγ, interferon-gamma.
The panels show one concept of GVHD prevention and/or therapy by prebiotic stabilization of the gut microbiome. (A) GVHD-associated disruption of gut mucosa homeostasis that is (partly) fueled by antibiotic-induced microbiome damage. (B) GOS prebiotics enhance the metabolic capacity of the microbiome to produce more butyrate and other SCFAs that can modulate alloreactivity and inflammatory processes in the allo-HCT recipient. APC, antigen-presenting cell; IFNγ, interferon-gamma.
In their study, Holmes et al used a dietary approach with GOS supplementation to prevent or reverse antibiotic-associated gut microbiome injury after allo-HCT. They found that administering GOS to mice treated with imipenem-cilastatin changed the composition of the microbiome with an expansion of butyrogenic bacteria (see figure panel B). Notably, in an in vitro fermentation model, the prebiotic also increased the fermentation capacity of the microbiome to produce more butyrate and other SCFAs; the results are summarized in panels A and B of the figure. However, whether the SCFA levels are increased in vivo in GOS-supplemented allo-HCT laboratory animals and, most importantly, how these metabolite changes affect alloreactive T-cell immunophenotypes in the host was not investigated in their study. Given that skin GVHD was more improved than gut GVHD in prebiotic-fed mice suggests that systemic microbial metabolite signaling rather than a change in the microbiome is confined to the gut lumen. It is also not clear why the administration of GOS failed to improve GVHD in allo-HCT mice that were not exposed to antibiotics.
Recently, a fiber-rich diet has been shown to facilitate the recovery of human gut microbiome diversity and metabolic output after healthy participants were exposed to antibiotics,8 which aligns with the increased metabolic output of the microbiome under GOS supplementation. Of note, Holmes et al demonstrated that the metabolic output of a prebiotic-conditioned microbiome depends on the composition of its species, as shown by the different effects of GOS supplementation in mice from Taconic Biosciences vs those from The Jackson Laboratory. These mice share the same genetic background, but they come with different background microbiomes because they were reared in different vivaria. This important observation highlights that dietary interventions with prebiotics need to be tailored to the gut microbiome of the individual as personalized nutrition.
We are now making the first steps toward understanding the rather complicated and highly individualized interactions among the diet, microbiome, and host in humans that have an impact on both immune function and cancer therapies.9,10 The Holmes et al study makes an important contribution by investigating the cross-talk of diet and microbiome in the allo-HCT and GVHD settings, which will help advance the field of dietary microbiome interventions in cohorts of high-risk patients with cancer.
Conflict-of-interest disclosure: C.K.S.-T. declares no competing financial interests.