In this issue of Blood, Vanden Bempt et al,1 using a well-designed mouse model, identify MYCN as a novel oncogenic driver in mature T-cell lymphoma. MYCN directly cooperates with enhancer of zeste homolog 2 (EZH2) and may represent a targetable mechanism implicated in the pathogenesis of T-cell lymphoma.
Human mature postthymic T-cell lymphomas are rare diseases (∼10% of lymphomas) but show enormous heterogeneity with more than 30 entities established by the recently published International Consensus Classification of Mature Lymphoid Tumors and 5th edition of World Health Organization Classification of Lymphoid Neoplasms.2,3 Many entities have well-characterized clinical, morphological, and genomic features, but ∼30% of all mature T-cell lymphomas remain unclassified, and thus are lumped into a single group (and most common type) known as peripheral T-cell lymphoma (PTCL), not otherwise specified (NOS).4
The rarity and heterogeneity of these neoplasms, as well as the lack of experimental models, have, until recently, limited the discovery of significant driver abnormalities, thereby impeding development of new therapeutic approaches. Anthracycline-based chemotherapy protocols, with or without autologous hematopoietic transplantation, have been the standard therapeutic approach for decades. Despite the recent addition of new agents, both overall survival and progression-free survival of most patients with PTCL remain dismal with urgent need of improvement.4
This situation is now changing with improvements in genomic testing that have identified significant defects that impact essential signaling pathways and foster T-cell transformation.5 Within the PTCL-NOS group, distinct subgroups have been identified, one of which is characterized by high expression of the transcription factor GATA3 and its target genes, as well as high MYC (and proliferation) gene expression signature that is associated with poor clinical outcome.6,7
The MYC family of oncogenes is deregulated in >50% of human cancers, frequently correlating with poor prognosis and unfavorable patient survival. The MYC family contains 3 members, MYC, MYCN, and MYCL, that encode MYC (also called MYCC), MYCN, and MYCL, respectively. MYC is a major regulator of the genome affecting 10% to 15% of all human genes. Many core cellular functions and pathways are under control of MYC, such as cell proliferation and growth, DNA replication, protein biosynthesis, and regulation of metabolism and energy. MYC is one of the most frequently disrupted genes in human lymphomas, as seen in the more common and well-described aggressive B-cell lymphomas.8 The critical oncogenic role of MYC has stimulated the search for therapeutic strategies that may counteract its damaging functions. Yet, MYC protein itself has so far been considered “undruggable.”8
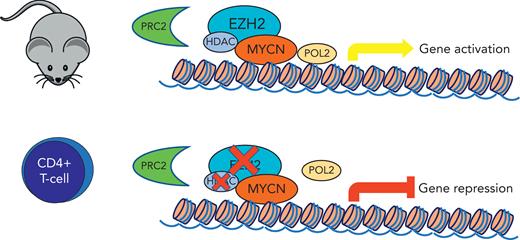
In the current study, Vanden Bempt et al focused their experiments on MYCN, a MYC paralogue often associated with neuroblastoma pathogenesis and rarely implicated in lymphoid neoplasms. Up to this point, the sole lymphoid neoplasm reported to involve MYCN was Burkitt lymphoma.9 The authors used a well-designed induced MYCN mouse model that promoted the development of CD4+ T-cell lymphomas (and, interestingly, B-cell lymphomas). Using transcriptomic and epigenetic approaches, they dissected the gene expression program modulated by MYCN and identify EZH2 as an essential transcriptional cofactor required to sustain MYCN activation, independent of the polycomb repressive complex 2 (PRC2) and linked to CDK1-mediated phosphorylation. These findings are in keeping with previous reports on the role of MYCN (as well as MYCC) and EZH2 in neuroblastoma, solid cancers, and Burkitt lymphoma cell lines.10
In the current study, MYCN-induced mice T-cell lymphoma cells were only slightly affected by inhibition of the EZH2 enzymatic activity but were sensitive to selective EZH2 degradation or CDK1 inhibition, which displayed synergy with US Food and Drug Administration–approved histone deacetylase inhibitors (see figure).
Induced MYCNexpression in CD4+ T-cell mouse model shows interaction with EZH2 through a noncanonical function independent of PRC2. MYCN activation is sensitive to selective EZH2 degradation, which was synergistic with histone deacetylase inhibitors.
Induced MYCNexpression in CD4+ T-cell mouse model shows interaction with EZH2 through a noncanonical function independent of PRC2. MYCN activation is sensitive to selective EZH2 degradation, which was synergistic with histone deacetylase inhibitors.
The induced MYCN mouse model data were correlated with MYC and MYCN expression data of human PTCL samples. Using a cohort of 28 human PTCL samples (the majority PTCL, NOS), the authors showed MYCN to be overexpressed in 5 of their 28 cases (18%) and in 13 of the 152 cases (9%) of the combined current study and publicly available PTCL datasets. Yet, half of all PTCL cases showed a high MYC signature, which means that MYCN was a significant player in less than half of the MYC-associated PTCL cases. Moreover, 2 of the 7 cases (29%) with MYCN overexpression showed a low expression of MYC signature. This suggests (like the rare reports of MYCN role in B-cell lymphoma) that MYCN has an oncogenic role in a small but most likely significant number of PTCL cases.
Validation of the MYCN relevance in human PTCL is certainly needed using larger cohorts of well-characterized PTCL samples. However, given the urgent need for an improved understanding of these gloomy lymphomas, this study by Vanden Bempt et al provides a relevant model that could be used as the basis for further investigations exploring combinatorial therapies in a small but significant subset of PTCL.
Conflict-of-interest disclosure: The author declares no competing financial interests.