Abstract
Multiple myeloma (MM) remains an incurable plasma cell malignancy that develops in the bone marrow (BM). This BM is partially responsible for protecting the MM cells against current standard-of-care therapies and for accommodating MM-related symptoms such as bone resorption and immune suppression. Increasing evidence has implicated extracellular vesicles (EV), including exosomes in the different processes within the BM. Exosomes are <150-nm-sized vesicles secreted by different cell types including MM cells. These vesicles contain protein and RNA cargo that they deliver to the recipient cell. In this way, they have been implicated in MM-related processes including osteolysis, angiogenesis, immune suppression, and drug resistance. Targeting exosome secretion could therefore potentially block these different processes. In this review, we will summarize the current findings of exosome-related processes in the BM and describe not only the current treatment strategies to counter them but also how exosomes can be harnessed to deliver toxic payloads. Finally, an overview of the different clinical studies that investigate EV cargo as potential MM biomarkers in liquid biopsies will be discussed.
Introduction
In multiple myeloma (MM), malignant plasma cells (PCs) expand within the bone marrow (BM), leading to typical symptoms such as bone lesions, anemia, hypercalcemia, and recurrent infections. The worldwide incidence is currently 160 000.1 MM is often preceded by a precancerous asymptomatic stage called monoclonal gammopathy of undetermined significance (MGUS). Fifteen percent of patients with MGUS evolve to MM, with or without an intermediate state of smoldering MM (SMM). Currently, the percentage of BM PCs is used to stratify patients with MM, whereby PCs are defined as CD138+/CD38+. However, CD138 can be shed from the membrane, indicating a need for novel biomarkers.2
Despite the development of novel treatment strategies, MM remains incurable; most MM patients relapse and become refractory to all therapies.3 The BM microenvironment is known to play a crucial role in both progression and relapse.4 Much effort has been invested in finding therapeutic targets by understanding niche-tumor crosstalk; however, this has mostly led to disappointing clinical impact. In recent years, a new communication mode has emerged, namely secreted extracellular vesicles (EVs). These EVs encompass a heterogeneous group of vesicles consisting of microvesicles (MVs), apoptotic bodies, exosomes, and exomeres, depending on their size and secretion pathway. Apoptotic bodies are the largest EVs (1-5 μm) that are released from the plasma membrane during late stages of apoptosis, whereas MVs are a heterogeneous group of vesicles with a size ranging from 100 nm to 1 μm, which bleb from the plasma membrane. Exomeres are small (<50 nm) nonmembranous vesicles.5
In this review, we mainly focus on the role of exosomes in MM development and therapy and their possible value as biomarkers.
Exosomes
Exosome biogenesis and uptake6
Exosomes are small vesicles (30-150 nm), defined by their endosomal origin. The process of exosome formation and uptake is described in detail in Figure 1. Briefly, early endosomes will mature into multivesicular bodies that will fuse with the plasma membrane to release their content as exosomes. Biogenesis of exosomes is either ESCRT (endosomal sorting complex required for transport) dependent, whereby ESCRT proteins form complexes with syndecans via syntenin,7 or it can be independently8 regulated by tetraspanins or the lipid ceramide.9 Exosome release is mediated by Rab GTPases and SNARE proteins.10,11 Their cargo includes lipids, proteins, small RNA, and possibly DNA.12
Exosome biogenesis and uptake. (A) [1] The process of exosome formation begins with the inward budding of the plasma membrane to form primary endocytic vesicles, which fuse together to create early endosomes. [2] Early endosomes mature into late endosomes at which point a second inward budding occurs to form intraluminal vesicles (ILVs). Late endosomes that contain several ILVs are called multivesicular bodies (MVBs). Biogenesis of exosomes can be either ESCRT (endosomal sorting complex required for transport) dependent or independent. ESCRT proteins (including TSG-101) can be divided in four multimeric complexes (ESCRT-0, -I, -II, -III) each with their defined role in vesicle formation. The accessory proteins Bro1/ALG-2-interacting protein X (ALIX) and vacuolar protein sorting (VPS4) ATPase are involved in stabilizing the complex. Syntenin is a multivalent protein that binds the cytosolic domain of syndecan but also directly interacts with ALIX. ESCRT independent exosome biogenesis involves lipids such as ceramide or tetraspanins. ESCRT dependent and independent mechanisms most likely also work together. [3] The last step in exosome biogenesis is their release into extracellular space. This step includes the transport of MVBs to the plasma membrane, followed by their docking and fusion. This process is regulated by proteins involved in cytoskeletal rearrangements and fusion machinery such as the Rab family of GTPases and the SNARE (soluble NSF attachment protein receptors) family proteins. Different Rab proteins have been implicated in vesicular trafficking of which Rab27a/b are best described. The SNARE protein family encompasses more than 60 members, which induce membrane fusion. The best described SNAREs involved in exosome release are VAMP3 and VAMP7. (B) Different uptake mechanisms have been described. [1] Specific uptake through receptors present on the membrane which is dependent on which ligands are expressed on the membrane of the exosomes. [2] Pinocytosis, during which actin-driven membrane ruffling is triggered in the recipient cell. Lamellipodia will form pinocytic cups in which exosomes, bound to the membrane, are “captured”. Once the pinocytic cups close, they are termed pinocytomes, which will shrink to the size of endosomes. [3] Clathrin-mediated endocytosis is a receptor mediated process whereby clathrin-coated vesicles will be formed, followed by invagination of the membrane and fusion to endosomes. Dynamin 2, clathrin and adaptor protein 2 (AP2) are the best characterized proteins involved in this process. Caveolin-dependent endocytosis is similar to clathrin but involves the presence of caveolae, small plasma membrane invaginations rich in caveolin, cholesterol and sphingolipids. [4] Plasma membrane fusion, during which the membrane of the exosome directly merges with the plasma membrane, releasing exosome content into the cytosol. Rab and SNARE family proteins contribute to this process. This figure was created with biorender.com.
Exosome biogenesis and uptake. (A) [1] The process of exosome formation begins with the inward budding of the plasma membrane to form primary endocytic vesicles, which fuse together to create early endosomes. [2] Early endosomes mature into late endosomes at which point a second inward budding occurs to form intraluminal vesicles (ILVs). Late endosomes that contain several ILVs are called multivesicular bodies (MVBs). Biogenesis of exosomes can be either ESCRT (endosomal sorting complex required for transport) dependent or independent. ESCRT proteins (including TSG-101) can be divided in four multimeric complexes (ESCRT-0, -I, -II, -III) each with their defined role in vesicle formation. The accessory proteins Bro1/ALG-2-interacting protein X (ALIX) and vacuolar protein sorting (VPS4) ATPase are involved in stabilizing the complex. Syntenin is a multivalent protein that binds the cytosolic domain of syndecan but also directly interacts with ALIX. ESCRT independent exosome biogenesis involves lipids such as ceramide or tetraspanins. ESCRT dependent and independent mechanisms most likely also work together. [3] The last step in exosome biogenesis is their release into extracellular space. This step includes the transport of MVBs to the plasma membrane, followed by their docking and fusion. This process is regulated by proteins involved in cytoskeletal rearrangements and fusion machinery such as the Rab family of GTPases and the SNARE (soluble NSF attachment protein receptors) family proteins. Different Rab proteins have been implicated in vesicular trafficking of which Rab27a/b are best described. The SNARE protein family encompasses more than 60 members, which induce membrane fusion. The best described SNAREs involved in exosome release are VAMP3 and VAMP7. (B) Different uptake mechanisms have been described. [1] Specific uptake through receptors present on the membrane which is dependent on which ligands are expressed on the membrane of the exosomes. [2] Pinocytosis, during which actin-driven membrane ruffling is triggered in the recipient cell. Lamellipodia will form pinocytic cups in which exosomes, bound to the membrane, are “captured”. Once the pinocytic cups close, they are termed pinocytomes, which will shrink to the size of endosomes. [3] Clathrin-mediated endocytosis is a receptor mediated process whereby clathrin-coated vesicles will be formed, followed by invagination of the membrane and fusion to endosomes. Dynamin 2, clathrin and adaptor protein 2 (AP2) are the best characterized proteins involved in this process. Caveolin-dependent endocytosis is similar to clathrin but involves the presence of caveolae, small plasma membrane invaginations rich in caveolin, cholesterol and sphingolipids. [4] Plasma membrane fusion, during which the membrane of the exosome directly merges with the plasma membrane, releasing exosome content into the cytosol. Rab and SNARE family proteins contribute to this process. This figure was created with biorender.com.
Exosomes can exert their function either by presenting molecules on their membrane or by releasing their cargo after internalization. The mechanisms behind EV uptake and cargo delivery are still poorly defined but include macro/micropinocytosis, clathrin-mediated endocytosis, caveolin-dependent endocytosis, plasma membrane fusion, and specific uptake through membrane receptors.13
Exosome markers and isolation techniques
Minimal information for studies of extracellular vesicles guidelines14 dictate that exosomes should be characterized by a minimum of 3 protein markers: 1 transmembranic protein (including CD63, CD9, and CD82) associated with the plasma membrane and/or endosomes, 1 cytosolic protein (including elements of the ESCRT such as TSG101 and/or ALIX) or flotillins (Flot 1 and 2) that associate with membrane microdomains, and 1 negative marker for other intracellular proteins such as calreticulin, calnexin, prohibitin, or GM130. HSP70, ARF6, and tubulin are also valid as cytosolic markers, whereas syntenin has been put forward as the most abundant protein in exosomes.15
A wide variety of isolation techniques14,16 is available to isolate exosomes, each with certain recovery/specificity. Precipitation kits such as Exoquick solutions or the ExoEasy kit are fast, accessible, and have a high yield but low specificity, making extra purification measures necessary. This technique should be approached with caution, especially for biomarker studies as it is nearly impossible to exclude coprecipitated cargo. Differential ultracentrifugation remains the most commonly used technique to isolate EVs17 and is considered to have intermediate specificity and yield but has as a drawback the need for heavy equipment that is not always available. Density gradient can further improve purity of exosome fractions by removing non-EV contaminants. Size exclusion chromatography is a valid alternative with similar characteristics. Combinations of different isolation techniques will increase specificity, which is necessary to detect specific exosomal cargo. One paper described that exosomes from serum from patients with MM, obtained by iodixanol and sucrose gradients, was pure, but those achieved with limited processing (serial centrifugation or 1-step precipitation kits) resulted in contamination by a residual matrix, embedding the exosomes.18 An overview of the different isolation techniques and markers for EV studies in MM are listed in Table 1.
Because most isolation techniques do not solely isolate exosomes, it has been proposed to use the term “small EVs” when considering exosomes. As characterization and functionality of exosomes/EVs are very method dependent, the International Society of Extracellular Vesicles has introduced EV-Track where authors can upload their manuscript to receive an EV-Metric score, based on components, which were argued to be indispensable for unambiguous interpretation and reproduction of EV experiments.19
Exosomes in myeloma pathogenesis
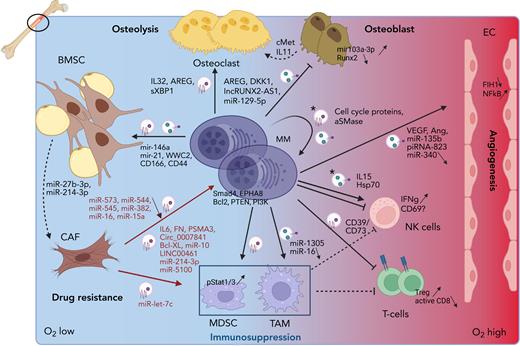
Increasing evidence has demonstrated that EVs, including exosomes, play a role in most of the BM-MM interactions and processes. These will be listed below and are depicted in Figure 2.
Role of EVs in the MM microenvironment. MM cells reside in the hypoxic BM milieu, which will trigger MM EV release. These EVs will stimulate angiogenesis by delivering pro-angiogenic cargo including VEGF, miR-135b and piRNA-823, which will trigger proliferation and tube formation in ECs. Osteolysis will be activated by inducing osteoclast proliferation and activation through the delivery of EV cargo (AREG, IL32 and sXBP1) and by blocking osteoblast differentiation and activity through inhibitory cargo such as AREG, DKK1, lncRUNX2-AS1, and miR-129-5p. Moreover, osteoblasts are stimulated to secrete cMet and IL11 for the osteoclasts. Immune suppression is induced by the presence of CD73 and CD39, and activation of MDSC and TAMs via miR-1305 EV delivery, who in turn will inhibit NK- and T-cell proliferation. MM EVs have a direct impact on T cells by inhibiting CD8 activation and inducing T regulatory cell expansion. Direct effects on NK cells are more inconclusive and only occur after treating MM cells with cytotoxic compounds, releasing “chemoexosomes” (depicted with a ∗). IFNγ is released in response to chemoexosomes, but NK activation is uncertain. These chemoexosomes also contain cell cycle proteins and acid SMase, thereby inducing auto-resistance. Finally, MM cells trigger BMSC to transform into CAFs by delivering mir-146a, mir-21, WWC2, CD166, and CD44 as EV cargo. Transition toward CAFs is accompanied by miR-27b-3p and miR-214-3p upregulation. CAFs in turn will release growth and survival cargo to MM cells, containing IL6, FN, PSMA3, Circ_0007841, Bcl-XL, miR-10, miR-214-3p, miR-5100 and LINC00461. They will also stimulate TAMs via delivery of miR-let-7c. A downward arrow indicates less expression of specific cargo in tumoral EVs. This figure was created with biorender.com.
Role of EVs in the MM microenvironment. MM cells reside in the hypoxic BM milieu, which will trigger MM EV release. These EVs will stimulate angiogenesis by delivering pro-angiogenic cargo including VEGF, miR-135b and piRNA-823, which will trigger proliferation and tube formation in ECs. Osteolysis will be activated by inducing osteoclast proliferation and activation through the delivery of EV cargo (AREG, IL32 and sXBP1) and by blocking osteoblast differentiation and activity through inhibitory cargo such as AREG, DKK1, lncRUNX2-AS1, and miR-129-5p. Moreover, osteoblasts are stimulated to secrete cMet and IL11 for the osteoclasts. Immune suppression is induced by the presence of CD73 and CD39, and activation of MDSC and TAMs via miR-1305 EV delivery, who in turn will inhibit NK- and T-cell proliferation. MM EVs have a direct impact on T cells by inhibiting CD8 activation and inducing T regulatory cell expansion. Direct effects on NK cells are more inconclusive and only occur after treating MM cells with cytotoxic compounds, releasing “chemoexosomes” (depicted with a ∗). IFNγ is released in response to chemoexosomes, but NK activation is uncertain. These chemoexosomes also contain cell cycle proteins and acid SMase, thereby inducing auto-resistance. Finally, MM cells trigger BMSC to transform into CAFs by delivering mir-146a, mir-21, WWC2, CD166, and CD44 as EV cargo. Transition toward CAFs is accompanied by miR-27b-3p and miR-214-3p upregulation. CAFs in turn will release growth and survival cargo to MM cells, containing IL6, FN, PSMA3, Circ_0007841, Bcl-XL, miR-10, miR-214-3p, miR-5100 and LINC00461. They will also stimulate TAMs via delivery of miR-let-7c. A downward arrow indicates less expression of specific cargo in tumoral EVs. This figure was created with biorender.com.
Angiogenesis
The MM burden increases the natural hypoxia of the BM,20 thereby triggering angiogenesis.21 Exosomes derived from hypoxia-resistant MM cells were found to contain high levels of the microRNA miR-135b that suppressed its target “factor–inhibiting hypoxia inducible factor 1” when transferred into endothelial cells (ECs), leading to their proliferation in vitro and in vivo.22 Our group demonstrated that MM exosomes induced EC proliferation and tube formation via Stat3 phosphorylation. Moreover, we identified multiple angiogenic factors as cargo proteins, including angiogenin, basic fibroblast growth factor, and vascular endothelial growth factor (VEGF).23 More recently, MM MVs were found to contain the small silencing piwi-interacting RNA piRNA-823, which when transferred to ECs, promoted proliferation, invasion, and tube formation by enhancing expression of VEGF, interleukin 6 (IL-6), and ICAM-1. piRNA-823–transfected ECs enhanced tumor growth when co-inoculated with MM cells in a xenograft model.24 Exosomes from MM patient serum were found to induce more proliferation in ECs compared with MGUS exosomes. This was associated with an altered intracellular c-Src distribution and activation of the nuclear factor κB (NF-κB) pathway.25 By contrast, exosomes derived from bone marrow stromal cells (BMSCs) isolated from healthy young people strongly inhibited angiogenesis. This was linked to high expression of miR-340, which could inhibit the c-Met signaling pathway.26 Interestingly, treating MM cells with C6 ceramide blocked tube formation by delivering increased levels of exosomal miR-29b that blocked the PI3K-Akt pathway in ECs.27 Treating MM cells with bortezomib also altered the composition of MM MVs, with more proinflammatory cytokines and less proangiogenic cytokines such as VEGF and angiogenin. Furthermore, these MVs inhibited NF-κB signaling in human umbilical vein endothelial cells leading to less proliferation and migration/tube formation.28,29
Osteolysis
MM cells trigger osteolysis by activating osteoclasts and inhibiting osteoblasts.30 MM exosomes were found to induce osteoclast formation and activity by activating the pAkt pathway.31 They contained activators of the unfolded protein response pathway, triggering IRE1α activation and downstream signaling.32 Also the presence of IL-32, which was upregulated in response to hypoxia, induced osteoclast activity both in vitro and in vivo.33 We further demonstrated that MM exosomes increased osteoclast formation and activity while simultaneously blocking osteoblast differentiation, inducing osteoblast apoptosis by downregulating Runx2, Osterix, and collagen 1A1 via the presence of DKK1 cargo. Injecting healthy mice with MM exosomes was sufficient to trigger bone disease.34 Similarly, amphiregulin was found to be present in MM exosomes, which could bind to epidermal growth factor receptor both in osteoclasts precursors and mesenchymal stem cells (MSCs), thereby activating the former and blocking the differentiation of the latter into osteoblasts.35 More recently it was discovered that miR-129-5p was upregulated in MM exosomes compared with SMM exosomes. This micro RNA (miRNA), once transferred to MSCs, inhibited the transcription factor Sp1 and its target alkaline phosphatase, thereby blocking osteoblast differentiation.36 MM exosomes could also block osteoblast differentiation from BMSC, by triggering IL-6 secretion from BMSCs via the Ape1/NF-κB pathway.37 In line with this, the presence of the long noncoding antisense RNA LncRUNX2-AS1 in human myeloma cell line (HMCL)-derived exosomes impaired osteogenic differentiation of MSCs by negatively regulating RUNX2 expression.38 Addition of MM MVs to MSC also induced elevated expression of miR-103a-3p, thereby inhibiting osteogenesis. Injecting MM MVs in a xenograft model further exacerbated MM bone disease.39 Finally, the presence of hepatocyte growth factor on exosomes of a subtype of patients with MM could induce c-Met signaling in osteoblast-like cells, thereby inducing the release of the osteoclast activating cytokine IL-11.40
Immunosuppressive environment
The BM environment in MM is immunosuppressive because of the presence of cells such as myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAM)s.41 Our group has demonstrated that MM- and MM BMSC-derived exosomes induced MDSC expansion and activation through pStat1 and 3 signaling, in vitro and in vivo. Treating healthy mice with MM exosomes increased the prevalence and activity of MDSCs, leading to inhibition of T-cell proliferation.23,42 Moreover, MVs isolated from the BM plasma of patients with MM, contained increased levels of the ectoenzymes CD39 and CD73, resulting in increased levels of adenosine, which can activate MDSC and inhibit T-cell function.43 Exosomes from HMCL, specifically with del13, could induce polarization of peripheral blood monocytes into M2 TAMs via the NF-κB pathway. These exosomes contained less miR-16, which was found to inhibit IKKa/B.44 Moreover, under hypoxia, MM cells were able to secrete increased amounts of exosomes containing, among others, miR-1305, which when transferred to THP1 monocytes, induced M2 polarization.45 Also, MM-MSC cells could stimulate polarization of PBMC-derived macrophages into M2 macrophages via the transfer of miR-let-7c.46 Available results are conflicting concerning the effect of MM exosomes on natural killer (NK) cells, which are activated by recognizing molecular patterns of ligands such as danger-associated molecular patterns or pathogen-associated molecular patterns. Two reports suggested that treatment of MM cells with low doses of genotoxic drugs such as melphalan induced a higher secretion of exosomes that could trigger NK proliferation, activation (CD69 expression), and interferon γ (IFNγ) release through the presentation of IL-15(RA)47 or via binding of HSP70 to TLR2.48 However, a more recent report indicated that MM exosomes could induce IFNγ release but also reduced NK cytotoxicity. Treating MM cells with either eicosapentaenoic or docosahexaenoic acid reduced the immunosuppressive effect while keeping the immune stimulatory response.49 Of note, in this report, the MM cells were not treated with a genotoxic drug, which could explain the different outcome. Finally, it was found that MM exosomes tended to induce apoptosis of CD4+ T cells derived from healthy donors (HDs) and patients with MM but increased viability in CD8+ T cells. However, the cytotoxic capacity of these CD8+ T cells was decreased. Furthermore, the viability of HD T regulatory cells increased, indicating that MM exosomes alter T-cell phenotype to a more suppressive profile.50
Drug resistance
Several reports have evaluated whether exosomes are involved in the protection of MM cells by the BM environment. Patient BM-MSC–derived exosomes were found to induce proliferation and survival of MM cells, whereas those of healthy BM-MSCs reduced MM viability. MM BM-MSCs contained lower levels of the tumor suppressor miR-15a and higher levels of fibronectin and IL-6.51 Similarly, we found that BMSC-derived exosomes induced MM proliferation and survival via activation of different prosurvival pathways including pAkt, p53, and JNK. More importantly, BMSC exosomes could induce bortezomib resistance by increasing Bcl2 levels.52 BMSC exosomes also contained caspase3-cleaved Bcl-XL, which not only conveyed antiapoptotic signals to recipient MM cells but was necessary for exosomal uptake.53 Furthermore, BMSC could increase MM cell proliferation via miR-10a transfer. Intriguingly, blocking exosome secretion using the S1P receptor modulator FTY720 induced BMSC apoptosis by an intracellular overload of miR-10a, indicating that both cell types respond differently to the presence of miR-10. PTEN and CDK6 were affected in BMSC, whereas SMAD4 seemed to be a target in MM cells. Treatment of a xenograft with FTY720 led to a reduction in the number of MM-BMSC and abolished their prosurvival effect on the MM cells.54 Further investigation into the content of BMSC exosomes revealed that miR-573, miR-544, miR-545, miR-382, miR-16 (all down), and miR-10a (up) were able to distinguish MM patient BMSCs from MGUS and HD. Target gene analysis suggested EPHA8 as a target in MM for miR-10a, whereas miR-16 negatively impacted cyclin D1 and IGFR1.55 MM BMSC exosomes also contained miR-23b-3p, miR-27b-3p, miR-125b-5p, miR-214-3p, and miR-5100. However, MM cells only incorporated miR-214-3p and miR-5100, which induced MM cell proliferation and drug resistance via downregulation of PTEN.56 Moreover, LINC00461, present in BM MSC exosomes, was discovered to be a sponge for miR-15a/16 after transfer into MM cells, thereby releasing BCL-2, leading to enhanced MM survival.57 Exosomal transfer of the circular RNA Circ_0007841 from BM-MSCs to MM cells could also induce survival, proliferation, and migration of MM cells via targeting of the miR-338-3p/BRD4 axis, which activated the PI3K/AKT signaling pathway.58 BMSCs were also found to have high levels of HDAC3 in the presence of MM cells, which in turn supported MM proliferation. Targeting HDAC3 in BMSC, induced changes in their exosome output both on a quantitative and qualitative level, including downregulation of prosurvival miR-380, miR-382, miR-15b, miR-9986, and miR-5191, which led to MM cell growth arrest.59 To further underscore the role of exosomes in drug resistance, it was found that exosomes derived from MSCs of proteasome inhibitor-resistant patients induced resistance in sensitive HMCL. PSMA3 mRNA and the long noncoding RNA (lncRNA) PSMA3-AS1, which codes for 1 of the proteasome subunits, was strongly present and increased proteasome activity after transfer to MM cells. Targeting PSMA3-AS1, reduced tumor burden in a xenograft model and synergized with carfilzomib treatment.60 It was further demonstrated that bortezomib-resistant MM cells could transfer resistance to sensitive cells via exosomal HSP70 delivery. Intravenous immunoglobulin G treatment, which contained different immunoglobulins including antibodies against HSP70, caused apoptosis of MM cells because of an interruption of this loop, thereby enhancing sensitivity to bortezomib both in vitro and in vivo.61
Not only the BM environment can induce drug resistance via exosomes, MM cells can also autologously induce therapy resistance. Treating MM cells with proteasome inhibitors led to increased secretion of exosomes, coined “chemoexosomes,” with an altered proteome profile, enriched in proteins that regulate cell cycle. Transfer of these exosomes to other MM cells enhanced proliferation and survival signals including pERK.62 Our group observed an increase in acid sphingomyelinase (ASM) expression in MM cell lines treated with melphalan or bortezomib, as well as in their exosomes. Exosomes high in ASM content were able to transfer the drug-resistant phenotype to chemosensitive cells, thereby suggesting a tumor-protective role for ASM.63 MM cells can also stimulate BMSCs to become cancer associated fibroblasts (CAFs) via exosomes, creating a mutual feedback loop. CAFs express fibroblast-specific protein 1 (FSP1) and, on activation by cancer cells, α-smooth muscle actin (αSMA) and fibroblast activation protein (FAP).64 Our group found that MM cells could transfer miR-146a via exosomes to MSCs, triggering through the NOTCH pathway enhanced cytokine secretion including IL-6 and CXCL10. This in turn stimulated MM growth and migration.65 MiR-146a transfer led to upregulation of αSMA and FAP in MSCs.66 MM exosomes further increased expression of miR-27b-3p and miR-214-3p in fibroblasts from patients with MGUS through transfer of exosomal WWC2, which regulates the Hippo pathway. These miRNAs modulated MCL1 via FBXW7 and PTEN and enhanced the expression of the CAF markers αSMA and FAP.67 Finally, when comparing the impact of HMCL derived exosomes to patient plasma-derived exosomes on healthy BMSCs, it was found that both were capable of inducing BMSC proliferation, whereas plasma exosomes also stimulated migration and adhesion to MM cells via the transfer of migration/adhesion related proteins (ie, MYH4, CD166, CD44, ANXA2, and FN1).68
Exosome therapies
Strategies to successfully block exosome secretion are still limited. We targeted exosome secretion using the sphingomyelinase inhibitor GW4869, which blocks the conversion of sphingomyelin into ceramide. This inhibitor reduced the release of exosomes, countered myeloma bone disease, and significantly enhanced the antitumor effect of bortezomib34 in the 5T33MM murine model. Another report indicated that GW4869 decreased exosome secretion from MM cells but decreased at the same time the level of EV-related tumor suppressive miRNAs (miR-202, 15a, 16, and 29b). By contrast, C6-ceramide treatment increased secretion of exosomes with a higher load of tumor suppressive miRNAs, leading to reduced viability in neighboring MM cells.69 Heparanase, an endoglycosidase present in the BM that cleaves heparan sulfate, is a possible target to block exosome release and uptake.70 Endosomal heparanase allows clustering of syndecans, by trimming heparin sulfate from syndecans, thereby recruiting them via ALIX to ESCRT.71 Exogenous heparanase could enhance exosome secretion of MM cells with increased levels of syndecan-1, VEGF, hepatocyte growth factor,72 and heparanase62 as exosomal cargo. These exosomes stimulated adhesion, ECM degradation and MM invasion in surrounding BM cells. On the receiving side, heparan sulfate chains were able to function as receptors for exosomes via mutual binding to fibronectin, after which exosomes were internalized, thereby activating downstream signaling pathways such as p38 and ERK. More importantly, the heparanase inhibitor Roneparstat, capable of inhibiting exosome binding to the cells,73 has been shown to have potent anti-MM activity in vivo74 and is currently being evaluated in a phase 1 clinical study in patients with advanced refractory myeloma.75 Related to this, overexpression of miR-1252-5p in MM cells was discovered to reduce heparanase levels. Accordingly, mimics of this miRNA, when electroporated into HEK293T cell MVs, led to enhanced bortezomib sensitivity in MM cells.76 Heparin treatment also reduced uptake of BMSC exosomes in MM cells by 50%, whereas inhibitors of clathrin- and caveolin-dependent endocytosis were at least equally effective. The dynamin inhibitor dynasore could block the protumoral effects of BMSC exosomes and synergized with bortezomib in a xenograft model.77
By contrast, exosomes can also be harnessed to present tumor antigens to the immune system. J558 myeloma cells were engineered to express the endogenous P1A tumor antigen and a transgenic form of membrane-bound HSP70, which served as an adjuvant danger signal. Exosomes from these cells could stimulate maturation of DCs in vitro and stimulate CD4+ T helper 1 and P1A-specific CD8+ cytotoxic T-lymphocyte responses in vivo.78 Apoptotic extracellular vesicles (apoEVs) derived from staurosporine-treated apoptotic MSCs could induce MM cell apoptosis by presenting FasL and triggering the trafficking of Fas to the surface of the cells. Treating 5TGM1 mice with apoEVs significantly prolonged their survival.79 Another study investigated the possibility of engineering tumor necrosis factor–related apoptosis-inducing ligand+ K562 exosomes. Although these exosomes were effective in inducing apoptosis in tumor necrosis factor–related apoptosis-inducing ligand death receptor (DR)5+ lymphoma cells, they were less potent in the DR5−DR4+KMS11 MM cell line.80 Intriguingly, exosomes can be armed with a payload to induce selective killing of target cells. The group of Kalluri has genetically engineered MSC exosomes (iExosomes) to carry short interfering RNA specific to oncogenic KrasG12D, which suppressed the growth of pancreatic cancer in different animal models.81 This study has kick-started a phase 1 clinical trial to evaluate dose and side effects of the iExosomes.82 Although no such study has been performed yet in MM, extrapolating these data to relevant MM oncogenes is a promising approach. Different myeloma targeting molecules such as anti-CD138 or anti-BCMA monoclonal antibodies could be engineered into the exosomes to direct them specifically to MM cells.
Exosome biomarkers
BM biopsies are still the gold standard to diagnose MM but have as a disadvantage that they are invasive and do not reflect spatial tumor heterogeneity. Liquid biopsies, whereby bodily fluids are used to isolate tumor material, have emerged as possible alternatives to circumvent these limitations. EVs, including exosomes, could serve as tumor material for biomarker discovery, either to diagnose patients with MM or to monitor them during treatment.83 An overview of the different biomarker trials can be found in Table 2.
Initial proteomic studies have focused on evaluating previously identified prognostic markers on MVs. Both the monoclonal immunoglobulin free light chain84 and the monoclonal immunoglobulin of the B-cell receptor have been demonstrated on MM-derived MVs.85 When examining PC markers, it was found that CD138 was more highly expressed in MM MVs compared with HD, which correlated to therapeutic response and disease stage.86,87 In a follow-up study, the multidrug resistance protein P-glycoprotein, together with the stem cell marker CD34 on CD138− MVs in patients with MM, correlated to unresponsiveness to treatment. This could be a consequence of CD138 shedding or an indication of the existence of a resistant immature phenotype.88 The levels of CD138+ MVs in the peripheral blood also correlated positively with the number of MM bone lesions.39 In addition, CD38+ MVs were more present in BM plasma and/or serum of patients with MM compared with patients with MGUS and SMM.43,89 Furthermore, using a simplified EV isolation technique that could be more easily implemented in routine clinical practice, the presence of CD38+, CD138+, and CD38+/CD138+ MVs was identified in patients with MM, whereby double-positive EVs correlated to BM-PC percentage.90 Other proteins such as MHC class I and CD44 were also found to be enriched in exosomes, derived either from MM cell lines or patient sera.91,92 Other cell types that are involved in MM progress could also release possible EV biomarkers. For example, CD163+ (hemoglobin-haptoglobin receptor) and CD206+ (mannose receptor) exosomes, derived from TAMs, were significantly elevated in patients with newly diagnosed MM compared with patients with HD, MGUS, and MM both in remission and relapse.93 As an alternative approach, patients could be stratified to either MM, MGUS, or asymptomatic MM, using either surface plasmon resonance spectroscopy or Raman spectroscopy to evaluate exosome levels.94,95
Concerning small RNA, a first study identified 22 miRNAs that were significantly lower in MM patient exosomes, among which let-7b and miR-18a, which were significant predictors for progression-free survival (PFS) and shorter overall survival (OS).96 In this study, downregulation of miR-155 was only reported to correlate with PFS; however, it was also lower in another small cohort of patients with MM.97 Similarly, several downregulated miRNA (including let-7c-5p, let-7d-5p, and miR-185-5p) and 2 upregulated miRNAs were found in MM exosome samples compared with SMM and HD.98 The same downregulated miRNAs in exosomes of patients with newly diagnosed MM had a strong negative correlation with disease progression, β2-microglobulin, and plasma cell load.99 Also the lncRNA PRINS was deregulated in exosomes of patients with newly diagnosed MM compared with MGUS and HD, and its expression had a negative correlation with PC percentage.100 When comparing exosomal miRNAs between responders and nonresponders to bortezomib, miR-17-5p, miR-20a-5p, miR-15a-5p, and miR-16-5p were lower in the resistant group.101 Similarly, 482 lncRNAs and 2099 mRNAs were discovered to be deregulated in exosomes of bortezomib-resistant patients. Of these, FFAR1 and SP9 were increased, whereas HIST1H2BG and ITIH2 were decreased. Exosomal FFAR1 and SP9 could be potential independent prognostic indicators of survival in patients with MM.102 Also, circMyc levels were higher in MM patient exosomes and correlated to 17p deletion and translocation of t(4;14). Moreover, circMyc levels were higher in bortezomib-resistant patients vs responders and were an independent predictor of poor prognosis.103
Concerning MM-induced pathologies, 265 upregulated circRNAs and 787 downregulated circular RNAs (circRNAs) in MM patient exosomes were screened for their impact on peripheral neuropathy. CircRNA chr2:2744228–2 744 407+ was found to be correlated with peripheral neuropathy characteristics and was predicted to downregulate miR-6829-3p and elevate GRIN2B.104 Similarly, exosomal circG042080 was identified as a possible predictor for MM-related myocardial damage.105 Moreover, a recent paper found that exosomal circATP10A in patients with MM had prognostic potential and correlated to vascular endothelial growth factor B protein levels, suggesting involvement in angiogenesis.106
Other possible EV cargos such as DNA or lipids have not been evaluated yet in MM. Although circulating cell-free DNA could serve as a possible biomarker, the presence of double-stranded DNA inside small EVs remains controversial, which can be attributed to discrepancies in the preparation method and size of the isolated EVs.107 Lipids are important players in a variety of processes including membrane formation and intracellular signaling. It would seem logical that lipid biomarkers can be evaluated in exosomes because the distribution of lipids is expected to be very similar to the plasma membrane. However, the evaluation of lipids in exosomes is not straightforward because of several risks including co-isolation of lipoproteins, poor lipid extraction, exclusion of lipophages, and the complex nature of biological membranes.108 Nevertheless, with standardized isolation and processing methods, these hurdles could be avoided, making EVs a valuable pool for lipidomic analysis.
Future directions and conclusion
It is now clear that EVs, including exosomes, have an established role in MM pathobiology by exchanging various cargo between the MM cells and their environment. Therefore, they provide attractive therapeutic targets to tackle MM-induced BM changes and DR. However, progress on this front is still disappointing because exosomes remain elusive potential targets. Unresolved issues include a lack of selectivity and insufficient insight into exosomal secretion routes as outlined in Table 3. These issues need to be addressed before exosomal targeting can move forward. Moreover, more knowledge of the different secretion routes could pinpoint a target that might be highly specific to the release of certain unwanted cargo. Because the use of exosomes as drug delivery vehicles is closer to clinical implementation, knowledge of these secretion pathways is also necessary to better understand how certain payloads can be incorporated into exosomes. In regard to the biomarker studies, there has been a clear rise in the number of MM-related studies, especially evaluating small RNA. However, there is no consensus yet on which is the best EV biomarker. For now, EV analysis should not replace biopsies but could be used to improve risk stratification. Unresolved issues for biomarker implementation include clinically feasible isolation procedures and interference by unassociated EV release. Moreover, the development of more sophisticated “omics” approaches will most likely fast-forward this domain.
In conclusion, the different studies reported in this review highlight the potential use of exosomes in MM, both as a therapeutic target and as potential material for liquid biopsies.
Authorship
Contribution: E.M. and K.V. wrote the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eline Menu, Department of Hematology and Immunology, Myeloma Center Brussels, Laarbeeklaan 103, B-1090 Brussels, Belgium; e-mail: eline.menu@vub.be.

![Exosome biogenesis and uptake. (A) [1] The process of exosome formation begins with the inward budding of the plasma membrane to form primary endocytic vesicles, which fuse together to create early endosomes. [2] Early endosomes mature into late endosomes at which point a second inward budding occurs to form intraluminal vesicles (ILVs). Late endosomes that contain several ILVs are called multivesicular bodies (MVBs). Biogenesis of exosomes can be either ESCRT (endosomal sorting complex required for transport) dependent or independent. ESCRT proteins (including TSG-101) can be divided in four multimeric complexes (ESCRT-0, -I, -II, -III) each with their defined role in vesicle formation. The accessory proteins Bro1/ALG-2-interacting protein X (ALIX) and vacuolar protein sorting (VPS4) ATPase are involved in stabilizing the complex. Syntenin is a multivalent protein that binds the cytosolic domain of syndecan but also directly interacts with ALIX. ESCRT independent exosome biogenesis involves lipids such as ceramide or tetraspanins. ESCRT dependent and independent mechanisms most likely also work together. [3] The last step in exosome biogenesis is their release into extracellular space. This step includes the transport of MVBs to the plasma membrane, followed by their docking and fusion. This process is regulated by proteins involved in cytoskeletal rearrangements and fusion machinery such as the Rab family of GTPases and the SNARE (soluble NSF attachment protein receptors) family proteins. Different Rab proteins have been implicated in vesicular trafficking of which Rab27a/b are best described. The SNARE protein family encompasses more than 60 members, which induce membrane fusion. The best described SNAREs involved in exosome release are VAMP3 and VAMP7. (B) Different uptake mechanisms have been described. [1] Specific uptake through receptors present on the membrane which is dependent on which ligands are expressed on the membrane of the exosomes. [2] Pinocytosis, during which actin-driven membrane ruffling is triggered in the recipient cell. Lamellipodia will form pinocytic cups in which exosomes, bound to the membrane, are “captured”. Once the pinocytic cups close, they are termed pinocytomes, which will shrink to the size of endosomes. [3] Clathrin-mediated endocytosis is a receptor mediated process whereby clathrin-coated vesicles will be formed, followed by invagination of the membrane and fusion to endosomes. Dynamin 2, clathrin and adaptor protein 2 (AP2) are the best characterized proteins involved in this process. Caveolin-dependent endocytosis is similar to clathrin but involves the presence of caveolae, small plasma membrane invaginations rich in caveolin, cholesterol and sphingolipids. [4] Plasma membrane fusion, during which the membrane of the exosome directly merges with the plasma membrane, releasing exosome content into the cytosol. Rab and SNARE family proteins contribute to this process. This figure was created with biorender.com.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/140/23/10.1182_blood.2021014749/4/m_blood_bld-2021-014749-c-gr1.jpeg?Expires=1767764476&Signature=1ntX76Fv75WskrQqOrhEyjXqFsP4mdXkSCbcnhZTN~USW37AacN0xv~47rBvIsR0~JRv3izgzyCuiCARq6pmRvpGcgUWVi4YiNZ3agoZPuTfukf8s4n05ctI6B~VLd9Xcw72saNnEGo1f43qVs0~3PIkRDvsTlt4CAsAXlbmt~U2TM~s4lQhtsihoZqcXZZGIm6yLCIS1zQpvLxEslvSmEUMIlfhm-Kvp3SMGgEEACpG2L4AE9sxy2LP-qekCVvO-Mc5BAqfpc3RSAO69b93CeMeSpynSofYL~Y7Nui52LtI9rrDaRSVSWxJ7pUNLUhhFhKFdRp3PerHFbVWiG5MGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)